Rational Design of a Novel Tubulin Inhibitor with a Unique Mechanism of Action
- PMID: 35404502
- PMCID: PMC9324959
- DOI: 10.1002/anie.202204052
Rational Design of a Novel Tubulin Inhibitor with a Unique Mechanism of Action
Abstract
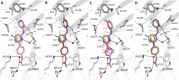
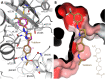
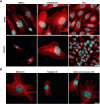
In this study, we capitalized on our previously performed crystallographic fragment screen and developed the antitubulin small molecule Todalam with only two rounds of straightforward chemical synthesis. Todalam binds to a novel tubulin site, disrupts microtubule networks in cells, arrests cells in G2/M, induces cell death, and synergizes with vinblastine. The compound destabilizes microtubules by acting as a molecular plug that sterically inhibits the curved-to-straight conformational switch in the α-tubulin subunit, and by sequestering tubulin dimers into assembly incompetent oligomers. Our results describe for the first time the generation of a fully rationally designed small molecule tubulin inhibitor from a fragment, which displays a unique molecular mechanism of action. They thus demonstrate the usefulness of tubulin-binding fragments as valuable starting points for innovative antitubulin drug and chemical probe discovery campaigns.
Keywords: Fragments; Microtubule-Targeting Agents; Molecular Mechanism of Action; Rational Drug Design; Tubulin.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


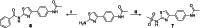


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

