Assessment of safety and immunogenicity of MHC homozygous iPSC-derived CD34+ hematopoietic progenitors in an NHP model
- PMID: 35404997
- PMCID: PMC9631690
- DOI: 10.1182/bloodadvances.2022006984
Assessment of safety and immunogenicity of MHC homozygous iPSC-derived CD34+ hematopoietic progenitors in an NHP model
Abstract
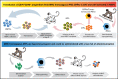
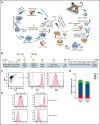
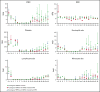
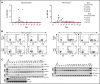
Administration of ex vivo expanded somatic myeloid progenitors has been explored as a way to facilitate a more rapid myeloid recovery and improve overall survival after myeloablation. Recent advances in induced pluripotent stem cell (iPSC) technologies have created alternative platforms for supplying off-the-shelf immunologically compatible myeloid progenitors, including cellular products derived from major histocompatibility complex (MHC) homozygous superdonors, potentially increasing the availability of MHC-matching cells and maximizing the utility of stem cell banking. However, the teratogenic and tumorigenic potential of iPSC-derived progenitor cells and whether they will induce alloreactive antibodies upon transfer remain unclear. We evaluated the safety and efficacy of using CD34+CD45+ hematopoietic progenitors derived from MHC homozygous iPSCs (iHPs) to treat cytopenia after myeloablative hematopoietic stem cell (HSC) transplantation in a Mauritian cynomolgus macaque (MCM) nonhuman primate (NHP) model. We demonstrated that infusion of iHPs was well tolerated and safe, observing no teratomas or tumors in the MCMs up to 1 year after HSC transplantation and iHP infusion. Importantly, the iHPs also did not induce significant levels of alloantibodies in MHC-matched or -mismatched immunocompetent MCMs, even after increasing MHC expression on iHPs with interferon-γ. These results support the feasibility of iHP use in the setting of myeloablation and suggest that iHP products pose a low risk of inducing alloreactive antibodies.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7(1):99-108. - PubMed
-
- Michels SL, Barron RL, Reynolds MW, Smoyer Tomic K, Yu J, Lyman GH. Costs associated with febrile neutropenia in the US. PharmacoEconomics. 2012;30(9):809-823. - PubMed
-
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015; 162(2):81-89. - PubMed
-
- Ozer H, Armitage JO, Bennett CL, et al. ; American Society of Clinical Oncology . 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 2000;18(20):3558-3585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

