The microRNA processor DROSHA is a candidate gene for a severe progressive neurological disorder
- PMID: 35405010
- PMCID: PMC9433733
- DOI: 10.1093/hmg/ddac085
The microRNA processor DROSHA is a candidate gene for a severe progressive neurological disorder
Abstract
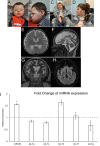
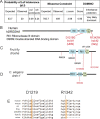
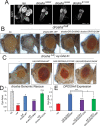
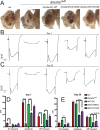
DROSHA encodes a ribonuclease that is a subunit of the Microprocessor complex and is involved in the first step of microRNA (miRNA) biogenesis. To date, DROSHA has not yet been associated with a Mendelian disease. Here, we describe two individuals with profound intellectual disability, epilepsy, white matter atrophy, microcephaly and dysmorphic features, who carry damaging de novo heterozygous variants in DROSHA. DROSHA is constrained for missense variants and moderately intolerant to loss-of-function (o/e = 0.24). The loss of the fruit fly ortholog drosha causes developmental arrest and death in third instar larvae, a severe reduction in brain size and loss of imaginal discs in the larva. Loss of drosha in eye clones causes small and rough eyes in adult flies. One of the identified DROSHA variants (p.Asp1219Gly) behaves as a strong loss-of-function allele in flies, while another variant (p.Arg1342Trp) is less damaging in our assays. In worms, a knock-in that mimics the p.Asp1219Gly variant at a worm equivalent residue causes loss of miRNA expression and heterochronicity, a phenotype characteristic of the loss of miRNA. Together, our data show that the DROSHA variants found in the individuals presented here are damaging based on functional studies in model organisms and likely underlie the severe phenotype involving the nervous system.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Fabian, M.R., Sonenberg, N. and Filipowicz, W. (2010) Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem., 79, 351–379. - PubMed
-
- Lau, N.C., Lim, L.P., Weinstein, E.G. and Bartel, D.P. (2001) An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science, 294, 858–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

