Predominantly Persistent Intraretinal Fluid in the Comparison of Age-related Macular Degeneration Treatments Trials
- PMID: 35405352
- PMCID: PMC10282893
- DOI: 10.1016/j.oret.2022.03.024
Predominantly Persistent Intraretinal Fluid in the Comparison of Age-related Macular Degeneration Treatments Trials
Abstract
Purpose: To describe predominantly persistent intraretinal fluid (PP-IRF) and its association with visual acuity (VA) and retinal anatomic findings at long-term follow-up in eyes treated with pro re nata (PRN) ranibizumab or bevacizumab for neovascular age-related macular degeneration.
Design: Cohort within a randomized clinical trial.
Participants: Participants in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) assigned to PRN treatment.
Methods: The presence of intraretinal fluid (IRF) on OCT scans was assessed at baseline and monthly follow-up visits by Duke OCT Reading Center. Predominantly persistent intraretinal fluid through week 12, year 1, and year 2 was defined as the presence of IRF at the baseline and in ≥ 80% of follow-up visits. Among eyes with baseline IRF, the mean VA scores (letters) and changes from the baseline were compared between eyes with and those without PP-IRF. Adjusted mean VA scores and changes from the baseline were also calculated using the linear regression analysis to account for baseline patient features identified as predictors of VA in previous CATT studies. Furthermore, outcomes were adjusted for concomitant predominantly persistent subretinal fluid.
Main outcome measures: Predominantly persistent intraretinal fluid through week 12, year 1, and year 2; VA score and VA change; and scar development at year 2.
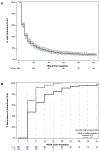
Results: Among 363 eyes with baseline IRF, 108 (29.8%) had PP-IRF through year 1 and 95 (26.1%) had PP-IRF through year 2. When eyes with PP-IRF through year 1 were compared with those without PP-IRF, the mean 1-year VA score was 62.4 and 68.5, respectively (P = 0.002), and was 65.0 and 67.4, respectively (P = 0.13), after adjustment. Predominantly persistent intraretinal fluid through year 2 was associated with worse adjusted 1-year mean VA scores (64.8 vs. 69.2; P = 0.006) and change (4.3 vs. 8.1; P = 0.01) as well as worse adjusted 2-year mean VA scores (63.0 vs. 68.3; P = 0.004) and changes (2.4 vs. 7.1; P = 0.009). Predominantly persistent intraretinal fluid through year 2 was associated with a higher 2-year risk of scar development (adjusted hazard ratio = 1.49; P = 0.03).
Conclusions: Approximately one quarter of eyes had PP-IRF through year 2. Predominantly persistent intraretinal fluid through year 1 was associated with worse long-term VA, but the relationship disappeared after adjustment for baseline predictors of VA. Predominantly persistent intraretinal fluid through year 2 was independently associated with worse long-term VA and scar development.
Trial registration: ClinicalTrials.gov NCT00593450.
Keywords: Anti-VEGF; Choroidal neovascularization; Intraretinal fluid; Persistent; Visual acuity.
Copyright © 2022 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
-
- Schmidt-Erfurth U, Waldstein SM, Deak GG, et al. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:822–832. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

