Lab-in-a-van: Rapid SARS-CoV-2 testing response with a mobile laboratory
- PMID: 35405388
- PMCID: PMC8989475
- DOI: 10.1016/j.ebiom.2022.103983
Lab-in-a-van: Rapid SARS-CoV-2 testing response with a mobile laboratory
Abstract
Background: High testing rates and rapid contact tracing have been key interventions to control COVID-19 in Victoria, Australia. A mobile laboratory (LabVan), for rapid SARS-CoV-2 diagnostics, was deployed at sites deemed critical by the Victorian State Department of Health as part of the response. We describe the process of design, implementation, and performance benchmarked against a central reference laboratory.
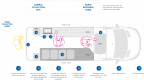
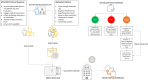
Methods: A BSL2 compliant laboratory, complete with a class II biological safety cabinet, was built within a Mercedes-Benz Sprinter Panel Van. Swabs were collected by on-site collection teams, registered using mobile internet-enabled tablets and tested using the Xpert® Xpress SARS-CoV-2 assay. Results were reported remotely via HL7 messaging to Public Health Units. Patients with negative results were automatically notified by mobile telephone text messaging (SMS).
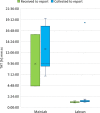
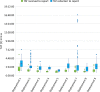
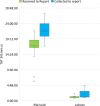
Findings: A pilot trial of the LabVan identified a median turnaround time (TAT) from collection to reporting of 1:19 h:mm (IQR 0:18, Range 1:03-18:32) compared to 9:40 h:mm (IQR 8:46, Range 6:51-19:30) for standard processing within the central laboratory. During deployment in nine rural and urban COVID-19 outbreaks the median TAT was 2:18 h:mm (IQR 1:18, Range 0:50-16:52) compared to 19:08 h:mm (IQR 5:49, Range 1:36-58:52) for samples submitted to the central laboratory. No quality control issues were identified in the LabVan.
Interpretation: The LabVan is an ISO15189 compliant testing facility fully operationalized for mobile point-of-care testing that significantly reduces TAT for result reporting, facilitating rapid public health actions.
Funding: This work was supported by the Department of Health, Victoria State Government, Australia.
Keywords: Mobile testing; Point-of-care; Rapid testing; Real-time PCR; SARS-CoV-2; Turnaround-time.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests AM and SY are employees of Department of Health, Victoria State Government and were involved with funding acquisition, project administration, resources for sample collection, manuscript drafting and revisions. They declare there is no conflict of interest. All remaining authors declare there are no conflicts of interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

