Real-world experience of abiraterone acetate plus prednisone in chemotherapy-naive patients with metastatic castration-resistant prostate cancer: long-term results of the prospective ABItude study
- PMID: 35405438
- PMCID: PMC9058899
- DOI: 10.1016/j.esmoop.2022.100431
Real-world experience of abiraterone acetate plus prednisone in chemotherapy-naive patients with metastatic castration-resistant prostate cancer: long-term results of the prospective ABItude study
Abstract
Background: Limited real-world data exist on the effectiveness and safety of abiraterone acetate plus prednisone (abiraterone hereafter) in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) naive to chemotherapy. Most of the few available studies had a retrospective design and included a small number of patients. In the interim analysis of the ABItude study, abiraterone showed good clinical effectiveness and safety profile in the chemotherapy-naive setting over a median follow-up of 18 months.
Patients and methods: We evaluated clinical and patient-reported outcomes (PROs) of chemotherapy-naive mCRPC patients treated with abiraterone as for clinical practice in the Italian, observational, prospective, multicentric ABItude study. mCRPC patients were enrolled at abiraterone start (February 2016-June 2017) and followed up for 3 years; clinical endpoints and PROs, including quality of life (QoL) and pain, were prospectively collected. Kaplan-Meier curves were estimated.
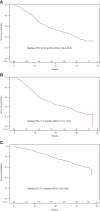
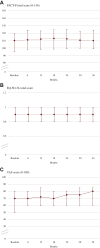
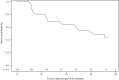
Results: Of the 481 patients enrolled, 454 were assessable for final study analyses. At abiraterone start, the median age was 77 years, with 58.6% elderly patients and 69% having at least one comorbidity (57.5% cardiovascular diseases). Visceral metastases were present in 8.4% of patients. Over a median follow-up of 24.8 months, median progression-free survival (any progression reported by the investigators), time to abiraterone discontinuation, and overall survival were, respectively, 17.3 months [95% confidence interval (CI) 14.1-19.4 months], 16.0 months (95% CI 13.1-18.2 months), and 37.3 months (95% CI 36.5 months-not estimable); 64.2% of patients achieved ≥50% reduction in prostate-specific antigen. QoL assessed by Functional Assessment of Cancer Therapy-Prostate, the European Quality of Life 5 Dimensions 3 Level, and European Quality of Life Visual Analog Scale remained stable during treatment. Median time to pain progression according to Brief Pain Inventory data was 31.1 months (95% CI 24.8 months-not estimable). Sixty-two patients (13.1%) had at least one adverse drug reaction (ADR) and 8 (1.7%) one serious ADR.
Conclusion: With longer follow-up, abiraterone therapy remains safe, well tolerated, and active in a large unselected population.
Keywords: abiraterone acetate; metastatic castration-resistant prostate cancer; prospective study; real-world evidence.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure GP has an honoraria/consulting or advisory role for AZ, Bayer, BMS, Ipsen, Janssen, Merk, MSD, Pfizer, and Novartis. VEC is an Advisory Board member and speaker for BMS, Ipsen, Janssen, and Pfizer. RB has an honoraria/consulting or advisory role/speaker’s bureau for Bayer, AstraZeneca, Sanofi, Novartis, Amgen, Roche, Pfizer, Janssen Cilag, and Bristol Mayer Squibb. SDP is an Advisory Board member and invited speaker for Novartis, Roche, Celgene, AstraZeneca, Amgen, Eisai, Lilly, Pfizer, and Gentili. PB is an employee of Janssen. The remaining authors have declared no conflicts of interest.
Figures
References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Miller K.D., Nogueira L., Mariotto A.B., et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. - PubMed
-
- Nuhn P., De Bono J.S., Fizazi K., et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate Ccncer in the era of precision oncology. Eur Urol. 2019;75(1):88–99. - PubMed
-
- Ritch C.R., Cookson M.S. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

