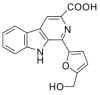
Flazin as a Lipid Droplet Regulator against Lipid Disorders
- PMID: 35406114
- PMCID: PMC9002757
- DOI: 10.3390/nu14071501
Flazin as a Lipid Droplet Regulator against Lipid Disorders
Abstract
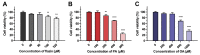
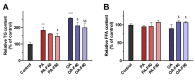
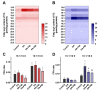
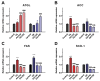
Lipid disorders are closely related to numerous metabolic diseases, and lipid droplets (LDs) have been considered as a new target for regulating lipid metabolism. Dietary intervention and nutraceuticals provide safe and long-term beneficial effects for treating metabolic diseases. Flazin is a diet-derived bioactive constituent mainly existing in fermented foods, of which the lipid metabolism improvement function has not been studied. In this study, the effect of flazin on lipid regulation at both cell level and organelle level was investigated. Lipidomic profiling showed that flazin significantly decreased cellular triglyceride (TG) by 12.0-22.4% compared with modeling groups and improved the TG and free fatty acid profile. LD staining revealed that flazin efficiently reduced both cellular neutral lipid content by 17.4-53.9% and LD size by 10.0-35.3%. Furthermore, nanoelectrospray ionization mass spectrometry analysis proved that flazin exhibited a preferential suppression of LD TG and regulated LD morphology, including a size decrease and surface property improvement. An evaluation of related gene expression suggested the mechanism to be lipolysis promotion and lipogenesis inhibition. These findings indicated that flazin might be an LD regulator for reversing lipid metabolism disturbance. Moreover, the strategy proposed in this study may contribute to developing other nutraceuticals for treating lipid disorder-related metabolic diseases.
Keywords: diabetic nephropathy; functional foods; lipid metabolism; lipid-storage disorders; lipidomics; mass spectrometry; metabolic diseases; nutraceuticals; triglyceride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


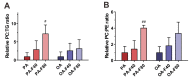

Similar articles
-
A unifying mathematical model of lipid droplet metabolism reveals key molecular players in the development of hepatic steatosis.FEBS J. 2017 Oct;284(19):3245-3261. doi: 10.1111/febs.14189. Epub 2017 Sep 6. FEBS J. 2017. PMID: 28763157
-
Formation, Identification, and Occurrence of the Furan-Containing β-Carboline Flazin Derived from l-Tryptophan and Carbohydrates.J Agric Food Chem. 2024 Mar 27;72(12):6575-6584. doi: 10.1021/acs.jafc.3c07773. Epub 2024 Mar 12. J Agric Food Chem. 2024. PMID: 38470992 Free PMC article.
-
Metabolism-lipid droplet-nucleic acid crosstalk to regulate lipid storage and other cellular processes in oleaginous Rhodococcus bacteria.Biol Cell. 2025 Jan;117(1):e2400094. doi: 10.1111/boc.202400094. Biol Cell. 2025. PMID: 39853774 Review.
-
CIDE family proteins control lipid homeostasis and the development of metabolic diseases.Traffic. 2020 Jan;21(1):94-105. doi: 10.1111/tra.12717. Traffic. 2020. PMID: 31746121 Review.
-
Flazin as a Promising Nrf2 Pathway Activator.J Agric Food Chem. 2019 Nov 20;67(46):12844-12853. doi: 10.1021/acs.jafc.9b04600. Epub 2019 Nov 12. J Agric Food Chem. 2019. PMID: 31668063
Cited by
-
Analyzing Active Compounds in Elateriospermum tapos Yogurt for Maternal Obesity: A Network Pharmacology and Molecular Docking Study.Foods. 2023 Sep 26;12(19):3575. doi: 10.3390/foods12193575. Foods. 2023. PMID: 37835227 Free PMC article.
-
Oxidative Stress and Lipid Dysregulation in Lipid Droplets: A Connection to Chronic Kidney Disease Revealed in Human Kidney Cells.Antioxidants (Basel). 2022 Jul 18;11(7):1387. doi: 10.3390/antiox11071387. Antioxidants (Basel). 2022. PMID: 35883878 Free PMC article.
References
-
- Moghadasian M.H., Kaur R., Kostal K., Joshi A.A., Molaei M., Le K., Fischer G., Bonomini F., Favero G., Rezzani R., et al. Anti-Atherosclerotic Properties of Wild Rice in Low-Density Lipoprotein Receptor Knockout Mice: The Gut Microbiome, Cytokines, and Metabolomics Study. Nutrients. 2019;11:2894. doi: 10.3390/nu11122894. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

