Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil
- PMID: 35406195
- PMCID: PMC9002719
- DOI: 10.3390/polym14071320
Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil
Abstract
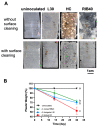
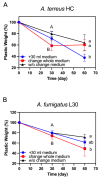
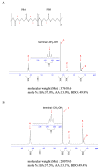
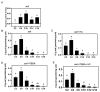
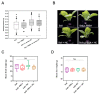
Plastic films are widely used in current agricultural practices; however, most mulch films used are discarded and buried in the land after harvest, having adverse environmental impacts. To solve this environmental problem, the demand for biodegradable mulch has been increasing in recent years. Polybutylene succinate-co-adipate (PBSA) is a biodegradable polymer with good ductility and can be used for packaging and mulching. In this study, we isolated two elite fungal strains for PBSA degradation from farmlands, i.e., Aspergillus fumigatus L30 and Aspergillus terreus HC, and the latter showed better degradation ability than the former. It is noteworthy that biodegradation of PBSA by A. terreus is reported for the first time, which revealed unique characteristics. In the soil burial test, even the soil with relatively poor degradation ability could be improved by the addition of elite fungal mycelia. In substrate specificity analyses of soil samples, PBSA could induce the synthesis of lipolytic enzymes of indigenous microbes to degrade substrates with medium and long carbon chains in soil. Furthermore, PBSA residues or fungal mycelia supplementation in soils had no adverse effect on the seed germination rate, seedling growth, or mature plant weight of the test green leafy vegetable. Taken together, the results of this study not only advance our understanding of the biodegradation of PBSA films by filamentous fungi but also provide insight into improving the efficiency of biodegradation in soil environments.
Keywords: Aspergillus; biodegradation; ecotoxicity; lipolytic enzyme; phytotoxicity; polybutylene succinate-co-adipate (PBSA).
Conflict of interest statement
The authors declare no conflict of interest. All authors have read and approved the final version of the manuscript.
Figures



References
-
- Bishop G., Styles D., Lens P.N. Recycling of European plastic is a pathway for plastic debris in the ocean. Environ. Int. 2020;142:105893. - PubMed
-
- Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

