Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium-Sulfur Batteries
- PMID: 35406216
- PMCID: PMC9002479
- DOI: 10.3390/polym14071342
Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium-Sulfur Batteries
Abstract
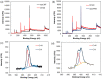
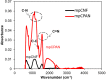
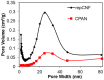
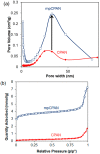
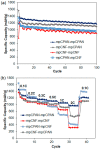
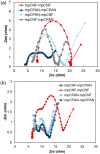
In the current work, we combined different physical and chemical modifications of carbon nanofibers through the creation of micro-, meso-, and macro-pores as well as the incorporation of nitrogen groups in cyclic polyacrylonitrile (CPAN) using gas-assisted electrospinning and air-controlled electrospray processes. We incorporated them into electrode and interlayer in Li-Sulfur batteries. First, we controlled pore size and distributions in mesoporous carbon fibers (mpCNF) via adding polymethyl methacrylate as a sacrificial polymer to the polyacrylonitrile carbon precursor, followed by varying activation conditions. Secondly, nitrogen groups were introduced via cyclization of PAN on mesoporous carbon nanofibers (mpCPAN). We compared the synergistic effects of all these features in cathode substrate and interlayer on the performance Li-Sulfur batteries and used various characterization tools to understand them. Our results revealed that coating CPAN on both mesoporous carbon cathode and interlayer greatly enhanced the rate capability and capacity retention, leading to the capacity of 1000 mAh/g at 2 C and 1200 mAh/g at 0.5 C with the capability retention of 88% after 100 cycles. The presence of nitrogen groups and mesopores in both cathodes and interlayers resulted in more effective polysulfide confinement and also show more promise for higher loading systems.
Keywords: Lithium–Sulfur batteries; air-controlled electrospray; gas assisted electrospinning; mesoporous carbon nanofiber; nitrogen doping.
Conflict of interest statement
There is no conflict to declare.
Figures








References
-
- Cañas N.A., Hirose K., Pascucci B., Wagner N., Friedrich K.A., Hiesgen R. Investigations of lithium-sulfur batteries using electrochemical impedance spectroscopy. Electrochim. Acta. 2013;97:42–51. doi: 10.1016/j.electacta.2013.02.101. - DOI
-
- Yin Y.-X., Xin S., Guo Y.-G., Wan L.-J. Lithium-Schwefel-Batterien: Elektrochemie, Materialien und Perspektiven. Angew. Chemie. 2013;125:13426–13441. doi: 10.1002/ange.201304762. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

