Posttranslational Modifications in Thyroid Cancer: Implications for Pathogenesis, Diagnosis, Classification, and Treatment
- PMID: 35406382
- PMCID: PMC8996999
- DOI: 10.3390/cancers14071610
Posttranslational Modifications in Thyroid Cancer: Implications for Pathogenesis, Diagnosis, Classification, and Treatment
Abstract
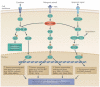
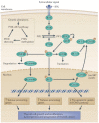
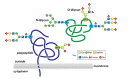
There is evidence that posttranslational modifications, including phosphorylation, acetylation, methylation, ubiquitination, sumoylation, glycosylation, and succinylation, may be involved in thyroid cancer. We review recent reports supporting a role of posttranslational modifications in the tumorigenesis of thyroid cancer, sensitivity to radioiodine and other types of treatment, the identification of molecular treatment targets, and the development of molecular markers that may become useful as diagnostic tools. An increased understanding of posttranslational modifications may be an important supplement to the determination of alterations in gene expression that has gained increasing prominence in recent years.
Keywords: acetylation; glycosylation; methylation; post-translational modifications; protein; succinylation; sumoylation; thyroid cancer; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Role of succinylation modification in thyroid cancer and breast cancer.Am J Cancer Res. 2021 Oct 15;11(10):4683-4699. eCollection 2021. Am J Cancer Res. 2021. PMID: 34765287 Free PMC article. Review.
-
Comprehensive review on Alzheimer's disease: From the posttranslational modifications of Tau to corresponding treatments.Ibrain. 2024 Sep 16;10(4):427-438. doi: 10.1002/ibra.12176. eCollection 2024 Winter. Ibrain. 2024. PMID: 39691421 Free PMC article. Review.
-
Assays for Posttranslational Modifications of Intermediate Filament Proteins.Methods Enzymol. 2016;568:113-38. doi: 10.1016/bs.mie.2015.09.005. Epub 2015 Nov 6. Methods Enzymol. 2016. PMID: 26795469 Free PMC article.
-
Posttranslational modification of the androgen receptor in prostate cancer.Int J Mol Sci. 2013 Jul 16;14(7):14833-59. doi: 10.3390/ijms140714833. Int J Mol Sci. 2013. PMID: 23863692 Free PMC article. Review.
-
Post-Translational Modifications of Transcription Factors Harnessing the Etiology and Pathophysiology in Colonic Diseases.Int J Mol Sci. 2020 May 1;21(9):3207. doi: 10.3390/ijms21093207. Int J Mol Sci. 2020. PMID: 32369982 Free PMC article. Review.
Cited by
-
Sirtuin 5-Mediated Desuccinylation of ALDH2 Alleviates Mitochondrial Oxidative Stress Following Acetaminophen-Induced Acute Liver Injury.Adv Sci (Weinh). 2024 Oct;11(39):e2402710. doi: 10.1002/advs.202402710. Epub 2024 Aug 19. Adv Sci (Weinh). 2024. PMID: 39159058 Free PMC article.
-
SIRT7 promotes the proliferation and migration of anaplastic thyroid cancer cells by regulating the desuccinylation of KIF23.BMC Cancer. 2024 Feb 15;24(1):210. doi: 10.1186/s12885-024-11965-9. BMC Cancer. 2024. PMID: 38360598 Free PMC article.
-
Ubiquitin-modifying enzymes in thyroid cancer:Mechanisms and functions.Heliyon. 2024 Jul 2;10(13):e34032. doi: 10.1016/j.heliyon.2024.e34032. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39091932 Free PMC article. Review.
-
In search of new stratification strategies: tissue proteomic profiling of papillary thyroid microcarcinoma in patients with localized disease and lateral neck metastases.J Cancer Res Clin Oncol. 2023 Dec;149(19):17405-17417. doi: 10.1007/s00432-023-05452-0. Epub 2023 Oct 20. J Cancer Res Clin Oncol. 2023. PMID: 37861757 Free PMC article.
-
Epigenetic Targets and Their Inhibitors in Thyroid Cancer Treatment.Pharmaceuticals (Basel). 2023 Apr 7;16(4):559. doi: 10.3390/ph16040559. Pharmaceuticals (Basel). 2023. PMID: 37111316 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

