A Review of the Current Impact of Inhibitors of Apoptosis Proteins and Their Repression in Cancer
- PMID: 35406442
- PMCID: PMC8996962
- DOI: 10.3390/cancers14071671
A Review of the Current Impact of Inhibitors of Apoptosis Proteins and Their Repression in Cancer
Abstract
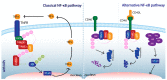
The Inhibitor of Apoptosis (IAP) family possesses the ability to inhibit programmed cell death through different mechanisms; additionally, some of its members have emerged as important regulators of the immune response. Both direct and indirect activity on caspases or the modulation of survival pathways, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), have been implicated in mediating its effects. As a result, abnormal expression of inhibitor apoptosis proteins (IAPs) can lead to dysregulated apoptosis promoting the development of different pathologies. In several cancer types IAPs are overexpressed, while their natural antagonist, the second mitochondrial-derived activator of caspases (Smac), appears to be downregulated, potentially contributing to the acquisition of resistance to traditional therapy. Recently developed Smac mimetics counteract IAP activity and show promise in the re-sensitization to apoptosis in cancer cells. Given the modest impact of Smac mimetics when used as a monotherapy, pairing of these compounds with other treatment modalities is increasingly being explored. Modulation of molecules such as tumor necrosis factor-α (TNF-α) present in the tumor microenvironment have been suggested to contribute to putative therapeutic efficacy of IAP inhibition, although published results do not show this consistently underlining the complex interaction between IAPs and cancer.
Keywords: NF-κB; SMAC mimetics; TNF-α; apoptosis; endoplasmic reticulum stress; inhibitor of apoptosis proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

