Reconstructive Surgery versus Primary Closure following Vulvar Cancer Excision: A Wide Single-Center Experience
- PMID: 35406468
- PMCID: PMC8997096
- DOI: 10.3390/cancers14071695
Reconstructive Surgery versus Primary Closure following Vulvar Cancer Excision: A Wide Single-Center Experience
Abstract
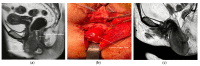
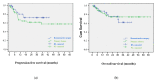
(1) Background: plastic reconstruction in vulvar surgery can lead to a better treatment outcome than primary closure. This study aims to compare the preoperative parameters (co-morbidities and tumor size) and postoperative results (tumor free margins and wound healing) between the primary closure and reconstructive surgery after vulvar cancer surgery; (2) Methods: this is a retrospective analysis of prospectively collected data from 2009 to 2021 at a tertiary cancer institution; (3) Results: 177 patients were included in the final analysis (51 patients had primary closure PC and 126 had reconstructive surgery RS). About half (49%) of the PC patients had no co-morbidities (p = 0.043). The RS group had a 45 mm median maximal tumor diameter compared to the PC group's 23 mm (p = 0.013). More than 90% of RS and 80% of PC had tumor-free margins (p = 0.1). Both groups had anterior vulvar excision as the most common surgery (52.4% RS vs. 23.5% PC; p = 0.001). Both groups had identical rates of wound healing disorders. In a median follow-up of 39 months; recurrent disease was found in 23.5% of PC vs. 10.3% in RS (p = 0.012). In terms of overall survival there was no significant difference between the both groups; (4) Conclusions: reconstructive vulvar surgery enables enhanced complete resection rates of larger vulvar tumors with better anatomical restoration and a comparable wound recovery in comparison to primary closure. This results in a lower recurrence rate despite the increased tumor volume.
Keywords: flap; primary closure; reconstructive surgery; vulvar cancer; vulvectomy; wound healing.
Conflict of interest statement
The first author, M.Z.M. is an adviser for BARD, Intuitive and Stryker. He received honoraria from Ethicon (Johnson and Johnson), Roche, and Astrazeneca in the last 2 years. J.S has received honoraria from AstraZeneca, Eisai, Clovis, Olympus, Johnson & Johnson, PharmaMar, Pfizer, TEVA, TESARO, and MSD, and performs advisory roles for AstraZeneca, Clovis, Lilly, PharmaMar, Pfizer, Roche, TESARO, and MSD. He has received research funding (not for this study) from AstraZeneca, Clovis, Merck, Bayer, PharmaMar, Pfizer, TESARO, and MSD. He has disclosed travel, accommodation, and other expenses paid or reimbursed by AstraZeneca, Clovis, PharmaMar, Roche, Pfizer, TESARO, and MSD, which were part of his scientific activities in the last 2 years. The other authors declare no conflict of interest.
Figures

References
-
- International Agency for Research on Cancer. [(accessed on 23 February 2022)]. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/21-Vulva-fact-sheet.pdf.
-
- Woelber L., Griebel L.F., Eulenburg C., Sehouli J., Jueckstock J., Hilpert F., de Gregorio N., Hasenburg A., Ignatov A., Hillemanns P., et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer-a subset analysis of the Arbeitsgemeinschaft Gynäkologische Onkologie CaRE-1 multicenter study. Eur. J. Cancer. 2016;69:180–188. doi: 10.1016/j.ejca.2016.09.038. - DOI - PubMed
-
- NCCN Guidelines Version 1.2022, Vulvar Cancer. [(accessed on 23 February 2022)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf.
LinkOut - more resources
Full Text Sources

