Energy Sources for Exosome Communication in a Cancer Microenvironment
- PMID: 35406470
- PMCID: PMC8996881
- DOI: 10.3390/cancers14071698
Energy Sources for Exosome Communication in a Cancer Microenvironment
Abstract
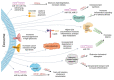
Exosomes are crucial extracellular vesicles (EVs) with a diameter of approximately 30-200 nm. They are released by most cell types in their extracellular milieu and carry various biomolecules, including proteins and nucleic acids. Exosomes are increasingly studied in various diseases, including cancer, due to their role in local and distant cell-cell communication in which they can promote tumor growth, cancer progression, and metastasis. Interestingly, a tremendous number of exosomes is released by malignant cancer cells, and these are then taken up by autologous and heterologous recipient stromal cells such as immune cells, cancer stem cells, and endothelial cells. All these events demand an enormous amount of energy and require that exosomes remain stable while having the capacity to reach distant sites and cross physical barriers. Nevertheless, there is a dearth of research pertaining to the energy sources of exosomes, and questions remain about how they maintain their motility in the tumor microenvironment (TME) and beyond. Moreover, exosomes can produce adenosine triphosphate (ATP), an important energy molecule required by all cells, and mitochondria have been identified as one of the exosomal cargoes. These findings strengthen the prospect of exosomal communication via transfer of mitochondria and the bioenergetics of target recipient cells. In the TME, the accumulation of ATP and lactate may facilitate the entry of exosomes into cancer cells to promote metastasis, as well as help to target cancer cells at the tumor site. This review highlights how exosomes obtain sufficient energy to thrive in the TME and communicate with distant physiological destinations.
Keywords: cancer; energy metabolism; exosome; extracellular vesicle; tumor microenvironment.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

References
-
- Qiu G., Thakur A., Xu C., Ng S.-P., Lee Y., Wu C.-M.L. Detection of Glioma-Derived Exosomes with the Biotinylated Antibody-Functionalized Titanium Nitride Plasmonic Biosensor. Adv. Funct. Mater. 2019;29:1806761. doi: 10.1002/adfm.201806761. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

