Immunomodulatory Properties of Immune Checkpoint Inhibitors-More than Boosting T-Cell Responses?
- PMID: 35406483
- PMCID: PMC8996886
- DOI: 10.3390/cancers14071710
Immunomodulatory Properties of Immune Checkpoint Inhibitors-More than Boosting T-Cell Responses?
Abstract
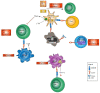
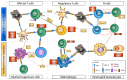
The approval of immune checkpoint inhibitors (ICI) that serve to enhance effector T-cell anti-tumor responses has strongly improved success rates in the treatment of metastatic melanoma and other tumor types. The currently approved ICI constitute monoclonal antibodies blocking cytotoxic T-lymphocyte-associated protein (CTLA)-4 and anti-programmed cell death (PD)-1. By this, the T-cell-inhibitory CTLA-4/CD80/86 and PD-1/PD-1L/2L signaling axes are inhibited. This leads to sustained effector T-cell activity and circumvents the immune evasion of tumor cells, which frequently upregulate PD-L1 expression and modulate immune checkpoint molecule expression on leukocytes. As a result, profound clinical responses are observed in 40-60% of metastatic melanoma patients. Despite the pivotal role of T effector cells for triggering anti-tumor immunity, mounting evidence indicates that ICI efficacy may also be attributable to other cell types than T effector cells. In particular, emerging research has shown that ICI also impacts innate immune cells, such as myeloid cells, natural killer cells and innate lymphoid cells, which may amplify tumoricidal functions beyond triggering T effector cells, and thus improves clinical efficacy. Effects of ICI on non-T cells may additionally explain, in part, the character and extent of adverse effects associated with treatment. Deeper knowledge of these effects is required to further develop ICI treatment in terms of responsiveness of patients to treatment, to overcome resistance to ICI and to alleviate adverse effects. In this review we give an overview into the currently known immunomodulatory effects of ICI treatment in immune cell types other than the T cell compartment.
Keywords: CTLA-4; PD-1; PD-L1; emerging immune checkpoints; immune checkpoint inhibitors; immunological effects; immunotherapy; metastatic melanoma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O., Patt D., Chen T.-T., Berman D.M., Wolchok J.D. Pooled Analysis of Long-Term Survival Data from Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

