Biomarker-Based Models for Preoperative Assessment of Adnexal Mass: A Multicenter Validation Study
- PMID: 35406551
- PMCID: PMC8997061
- DOI: 10.3390/cancers14071780
Biomarker-Based Models for Preoperative Assessment of Adnexal Mass: A Multicenter Validation Study
Abstract
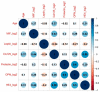
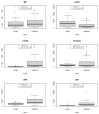
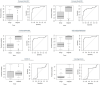
Ovarian cancer (OC) is the most lethal genital malignancy in women. We aimed to develop and validate new proteomic-based models for non-invasive diagnosis of OC. We also compared them to the modified Risk of Ovarian Malignancy Algorithm (ROMA-50), the Copenhagen Index (CPH-I) and our earlier Proteomic Model 2017. Biomarkers were assessed using bead-based multiplex technology (Luminex®) in 356 women (250 with malignant and 106 with benign ovarian tumors) from five European centers. The training cohort included 279 women from three centers, and the validation cohort 77 women from two other centers. Of six previously studied serum proteins (CA125, HE4, osteopontin [OPN], prolactin, leptin, and macrophage migration inhibitory factor [MIF]), four contributed significantly to the Proteomic Model 2021 (CA125, OPN, prolactin, MIF), while leptin and HE4 were omitted by the algorithm. The Proteomic Model 2021 revealed a c-index of 0.98 (95% CI 0.96, 0.99) in the training cohort; however, in the validation cohort it only achieved a c-index of 0.82 (95% CI 0.72, 0.91). Adding patient age to the Proteomic Model 2021 constituted the Combined Model 2021, with a c-index of 0.99 (95% CI 0.97, 1) in the training cohort and a c-index of 0.86 (95% CI 0.78, 0.95) in the validation cohort. The Full Combined Model 2021 (all six proteins with age) yielded a c-index of 0.98 (95% CI 0.97, 0.99) in the training cohort and a c-index of 0.89 (95% CI 0.81, 0.97) in the validation cohort. The validation of our previous Proteomic Model 2017, as well as the ROMA-50 and CPH-I revealed a c-index of 0.9 (95% CI 0.82, 0.97), 0.54 (95% CI 0.38, 0.69) and 0.92 (95% CI 0.85, 0.98), respectively. In postmenopausal women, the three newly developed models all achieved a specificity of 1.00, a positive predictive value (PPV) of 1.00, and a sensitivity of >0.9. Performance in women under 50 years of age (c-index below 0.6) or with normal CA125 (c-index close to 0.5) was poor. CA125 and OPN had the best discriminating power as single markers. In summary, the CPH-I, the two combined 2021 Models, and the Proteomic Model 2017 showed satisfactory diagnostic accuracies, with no clear superiority of either model. Notably, although combining values of only four proteins with age, the Combined Model 2021 performed comparably to the Full Combined Model 2021. The models confirmed their exceptional diagnostic performance in women aged ≥50. All models outperformed the ROMA-50.
Keywords: CA125; Copenhagen Index; Luminex; adnexal mass; diagnostic accuracy; multiplex; ovarian cancer; predictive models; tumor marker.
Conflict of interest statement
T.V.G., research support, advisory board, honoraria and travel expenses from AstraZeneca, Eisai Europe, GSK, Immunogen, MSD, OncXerna Therapeutics, PharmaMar, Roche; I.V., research support, advisory board, honoraria and travel expenses from Agenus, Aksebio, Amgen, AstraZeneca, Bristol Myers Squibb, Clovis Oncology Inc., Carrick Therapeutics, Deciphera Pharmaceuticals, Eisai, Elevar Therapeutics, F. Hoffmann-La Roche Ltd., Genmab, GSK, Immunogen Inc., Jazzpharma, Karyopharm, Mersana, Millennium Pharmaceuticals, MSD, Novocure, Novartis, Octimet Oncology NV, Oncoinvent AS, Roche, Seagen, Sotio a.s., Tesaro, Verastem Oncology, Zentalis; E.I.B., Bayer, Roche Diagnostics, Tesaro, GSK, AstraZeneca and personal fees from AstraZeneca, Clovis, GSK, Tesaro, EISAI, Roche Pharma, Roche Diagnostics. All other authors declare no conflict of interest.
Figures


References
-
- Global Burden of Disease 2019 Cancer Collaboration. Kocarnik J.M., Compton K., Dean F.E., Fu W., Gaw B.L., Harvey J.D., Henrikson H.J., Lu D., Pennini A., et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

