Neuroendocrine Neoplasms of the Gynecologic Tract
- PMID: 35406607
- PMCID: PMC8998008
- DOI: 10.3390/cancers14071835
Neuroendocrine Neoplasms of the Gynecologic Tract
Abstract
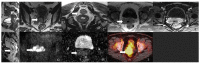
Gynecological tract neuroendocrine neoplasms (NEN) are rare, aggressive tumors from endocrine cells derived from the neuroectoderm, neural crest, and endoderm. The primary gynecologic NENs constitute 2% of gynecologic malignancies, and the cervix is the most common site of NEN in the gynecologic tract. The updated WHO classification of gynecologic NEN is based on the Ki-67 index, mitotic index, and tumor characteristics such as necrosis, and brings more uniformity in the terminology of NENs like other disease sites. Imaging plays a crucial role in the staging, triaging, restaging, and surveillance of NENs. The expression of the somatostatin receptors on the surface of neuroendocrine cells forms the basis of increasing evaluation with functional imaging modalities using traditional and new tracers, including 68Ga-DOTA-Somatostatin Analog-PET/CT. Management of NENs involves a multidisciplinary approach. New targeted therapies could improve the paradigm of care for these rare malignancies. This article focuses on the updated staging classifications, clinicopathological characteristics, imaging, and management of gynecologic NENs of the cervix, ovary, endometrium, vagina, and vulva, emphasizing the relatively common cervical neuroendocrine carcinomas among these entities.
Keywords: FIGO classification; PET/CT; cervical neuroendocrine tumor; gynecological NENs; imaging of neuroendocrine tumors; neuroendocrine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
Publication types
LinkOut - more resources
Full Text Sources

