Neutralization of GDF15 Prevents Anorexia and Weight Loss in the Monocrotaline-Induced Cardiac Cachexia Rat Model
- PMID: 35406637
- PMCID: PMC8997866
- DOI: 10.3390/cells11071073
Neutralization of GDF15 Prevents Anorexia and Weight Loss in the Monocrotaline-Induced Cardiac Cachexia Rat Model
Abstract
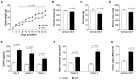
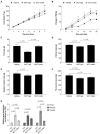
Growth and differentiation factor 15 (GDF15) is a cytokine reported to cause anorexia and weight loss in animal models. Neutralization of GDF15 was efficacious in mitigating cachexia and improving survival in cachectic tumor models. Interestingly, elevated circulating GDF15 was reported in patients with pulmonary arterial hypertension and heart failure, but it is unclear whether GDF15 contributes to cachexia in these disease conditions. In this study, rats treated with monocrotaline (MCT) manifested a progressive decrease in body weight, food intake, and lean and fat mass concomitant with elevated circulating GDF15, as well as development of right-ventricular dysfunction. Cotreatment of GDF15 antibody mAb2 with MCT prevented MCT-induced anorexia and weight loss, as well as preserved lean and fat mass. These results indicate that elevated GDF15 by MCT is causal to anorexia and weight loss. GDF15 mAb2 is efficacious in mitigating MCT-induced cachexia in vivo. Furthermore, the results suggest GDF15 inhibition is a potential therapeutic approach to alleviate cardiac cachexia in patients.
Keywords: GDF15; cardiac cachexia; monocrotaline.
Conflict of interest statement
B.A., X.C., D.H.-S., Y.Z., J.C.S., B.B.Z., A.S. and Z.W. are employees and shareholders of Pfizer.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

