Artificial Oocyte: Development and Potential Application
- PMID: 35406698
- PMCID: PMC8998074
- DOI: 10.3390/cells11071135
Artificial Oocyte: Development and Potential Application
Abstract
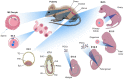
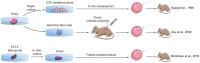
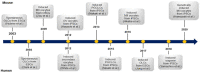
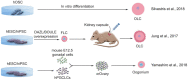
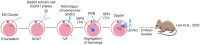
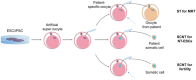
Millions of people around the world suffer from infertility, with the number of infertile couples and individuals increasing every year. Assisted reproductive technologies (ART) have been widely developed in recent years; however, some patients are unable to benefit from these technologies due to their lack of functional germ cells. Therefore, the development of alternative methods seems necessary. One of these methods is to create artificial oocytes. Oocytes can be generated in vitro from the ovary, fetal gonad, germline stem cells (GSCs), ovarian stem cells, or pluripotent stem cells (PSCs). This approach has raised new hopes in both basic research and medical applications. In this article, we looked at the principle of oocyte development, the landmark studies that enhanced our understanding of the cellular and molecular mechanisms that govern oogenesis in vivo, as well as the mechanisms underlying in vitro generation of functional oocytes from different sources of mouse and human stem cells. In addition, we introduced next-generation ART using somatic cells with artificial oocytes. Finally, we provided an overview of the reproductive application of in vitro oogenesis and its use in human fertility.
Keywords: artificial oocyte; assisted reproductive technologies; haploidization of somatic chromosomes; oogenesis; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

