Biotechnological Perspectives to Combat the COVID-19 Pandemic: Precise Diagnostics and Inevitable Vaccine Paradigms
- PMID: 35406746
- PMCID: PMC8997755
- DOI: 10.3390/cells11071182
Biotechnological Perspectives to Combat the COVID-19 Pandemic: Precise Diagnostics and Inevitable Vaccine Paradigms
Abstract
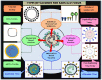
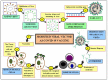
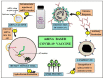
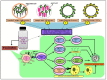
The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause for the ongoing global public health emergency. It is more commonly known as coronavirus disease 2019 (COVID-19); the pandemic threat continues to spread aroundthe world with the fluctuating emergence of its new variants. The severity of COVID-19 ranges from asymptomatic to serious acute respiratory distress syndrome (ARDS), which has led to a high human mortality rate and disruption of socioeconomic well-being. For the restoration of pre-pandemic normalcy, the international scientific community has been conducting research on a war footing to limit extremely pathogenic COVID-19 through diagnosis, treatment, and immunization. Since the first report of COVID-19 viral infection, an array of laboratory-based and point-of-care (POC) approaches have emerged for diagnosing and understanding its status of outbreak. The RT-PCR-based viral nucleic acid test (NAT) is one of the rapidly developed and most used COVID-19 detection approaches. Notably, the current forbidding status of COVID-19 requires the development of safe, targeted vaccines/vaccine injections (shots) that can reduce its associated morbidity and mortality. Massive and accelerated vaccination campaigns would be the most effective and ultimate hope to end the COVID-19 pandemic. Since the SARS-CoV-2 virus outbreak, emerging biotechnologies and their multidisciplinary approaches have accelerated the understanding of molecular details as well as the development of a wide range of diagnostics and potential vaccine candidates, which are indispensable to combating the highly contagious COVID-19. Several vaccine candidates have completed phase III clinical studies and are reported to be effective in immunizing against COVID-19 after their rollout via emergency use authorization (EUA). However, optimizing the type of vaccine candidates and its route of delivery that works best to control viral spread is crucial to face the threatening variants expected to emerge over time. In conclusion, the insights of this review would facilitate the development of more likely diagnostics and ideal vaccines for the global control of COVID-19.
Keywords: ARDS; COVID-19; CRISPR; SARS-CoV-2; coronavirus; detection methods; nucleic acid test (NAT); vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Worldometer Coronavirus. 2020. [(accessed on 8 November 2021)]. Available online: https://www.worldometers.info/coronavirus/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

