Myasthenia Gravis: An Acquired Interferonopathy?
- PMID: 35406782
- PMCID: PMC8997999
- DOI: 10.3390/cells11071218
Myasthenia Gravis: An Acquired Interferonopathy?
Abstract
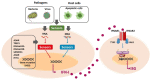
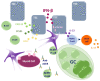
Myasthenia gravis (MG) is a rare autoimmune disease mediated by antibodies against components of the neuromuscular junction, particularly the acetylcholine receptor (AChR). The thymus plays a primary role in AChR-MG patients. In early-onset AChR-MG and thymoma-associated MG, an interferon type I (IFN-I) signature is clearly detected in the thymus. The origin of this chronic IFN-I expression in the thymus is not yet defined. IFN-I subtypes are normally produced in response to viral infection. However, genetic diseases called interferonopathies are associated with an aberrant chronic production of IFN-I defined as sterile inflammation. Some systemic autoimmune diseases also share common features with interferonopathies. This review aims to analyze the pathogenic role of IFN-I in these diseases as compared to AChR-MG in order to determine if AChR-MG could be an acquired interferonopathy.
Keywords: adaptive immunity; autoimmunity; germinal center; innate immunity; interferon type I; myasthenia gravis; pathogen infection; sterile inflammation; thymoma; thymus.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
-
- Cortés-Vicente E., Álvarez-Velasco R., Segovia S., Paradas C., Casasnovas C., Guerrero-Sola A., Pardo J., Ramos-Fransi A., Sevilla T., de Munain A.L., et al. Clinical and Therapeutic Features of Myasthenia Gravis in Adults Based on Age at Onset. Neurology. 2020;94:e1171–e1180. doi: 10.1212/WNL.0000000000008903. - DOI - PMC - PubMed
-
- Cho E.B., Min J.-H., Lee S., Yoon C.W., Seok J.M., Cho J., Lee H.L., Kim B.J. Late-Onset Non-Thymomatous Myasthenia Gravis: Comparison with Early-Onset and Very Late-Onset Myasthenia Gravis. Neurol. Asia. 2017;22:123–131.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

