The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke
- PMID: 35406804
- PMCID: PMC8997586
- DOI: 10.3390/cells11071239
The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke
Abstract
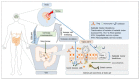
Recent research on the gut microbiome has revealed the influence of gut microbiota (GM) on ischemic stroke pathogenesis and treatment outcomes. Alterations in the diversity, abundance, and functions of the gut microbiome, termed gut dysbiosis, results in dysregulated gut-brain signaling, which induces intestinal barrier changes, endotoxemia, systemic inflammation, and infection, affecting post-stroke outcomes. Gut-brain interactions are bidirectional, and the signals from the gut to the brain are mediated by microbially derived metabolites, such as trimethylamine N-oxide (TMAO) and short-chain fatty acids (SCFAs); bacterial components, such as lipopolysaccharide (LPS); immune cells, such as T helper cells; and bacterial translocation via hormonal, immune, and neural pathways. Ischemic stroke affects gut microbial composition via neural and hypothalamic-pituitary-adrenal (HPA) pathways, which can contribute to post-stroke outcomes. Experimental and clinical studies have demonstrated that the restoration of the gut microbiome usually improves stroke treatment outcomes by regulating metabolic, immune, and inflammatory responses via the gut-brain axis (GBA). Therefore, restoring healthy microbial ecology in the gut may be a key therapeutic target for the effective management and treatment of ischemic stroke.
Keywords: cerebral stroke; gut dysbiosis; gut immune cells; gut leakiness; gut microbiota; gut-derived metabolites; gut–brain axis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
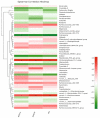
- Yamashiro K., Tanaka R., Urabe T., Ueno Y., Yamashiro Y., Nomoto K., Takahashi T., Tsuji H., Asahara T., Hattori N. Gut Dysbiosis Is Associated with Metabolism and Systemic Inflammation in Patients with Ischemic Stroke. PLoS ONE. 2017;12:e0171521. doi: 10.1371/journal.pone.0171521. - DOI - PMC - PubMed
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

