Proteomic Analysis of the Role of the Adenylyl Cyclase-cAMP Pathway in Red Blood Cell Mechanical Responses
- PMID: 35406814
- PMCID: PMC8997765
- DOI: 10.3390/cells11071250
Proteomic Analysis of the Role of the Adenylyl Cyclase-cAMP Pathway in Red Blood Cell Mechanical Responses
Abstract
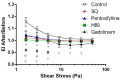
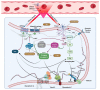
Red blood cell (RBC) deformability is modulated by the phosphorylation status of the cytoskeletal proteins that regulate the interactions of integral transmembrane complexes. Proteomic studies have revealed that receptor-related signaling molecules and regulatory proteins involved in signaling cascades are present in RBCs. In this study, we investigated the roles of the cAMP signaling mechanism in modulating shear-induced RBC deformability and examined changes in the phosphorylation of the RBC proteome. We implemented the inhibitors of adenylyl cyclase (SQ22536), protein kinase A (H89), and phosphodiesterase (PDE) (pentoxifylline) to whole blood samples, applied 5 Pa shear stress (SS) for 300 s with a capillary tubing system, and evaluated RBC deformability using a LORRCA MaxSis. The inhibition of signaling molecules significantly deteriorated shear-induced RBC deformability (p < 0.05). Capillary SS slightly increased the phosphorylation of RBC cytoskeletal proteins. Tyrosine phosphorylation was significantly elevated by the modulation of the cAMP/PKA pathway (p < 0.05), while serine phosphorylation significantly decreased as a result of the inhibition of PDE (p < 0.05). AC is the core element of this signaling pathway, and PDE works as a negative feedback mechanism that could have potential roles in SS-induced RBC deformability. The cAMP/PKA pathway could regulate RBC deformability during capillary transit by triggering significant alterations in the phosphorylation state of RBCs.
Keywords: capillary transit; cytoskeletal proteins; phosphorylation; red blood cell deformability; shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

