Transport Properties of Methyl-Terminated Germanane Microcrystallites
- PMID: 35407246
- PMCID: PMC9000464
- DOI: 10.3390/nano12071128
Transport Properties of Methyl-Terminated Germanane Microcrystallites
Abstract
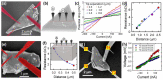
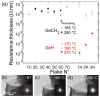
Germanane is a two-dimensional material consisting of stacks of atomically thin germanium sheets. It's easy and low-cost synthesis holds promise for the development of atomic-scale devices. However, to become an electronic-grade material, high-quality layered crystals with good chemical purity and stability are needed. To this end, we studied the electrical transport of annealed methyl-terminated germanane microcrystallites in both high vacuum and ultrahigh vacuum. Scanning electron microscopy of crystallites revealed two types of behavior which arise from the difference in the crystallite chemistry. While some crystallites are hydrated and oxidized, preventing the formation of good electrical contact, the four-point resistance of oxygen-free crystallites was measured with multiple tips scanning tunneling microscopy, yielding a bulk transport with resistivity smaller than 1 Ω·cm. When normalized by the crystallite thickness, the resistance compares well with the resistance of hydrogen-passivated germanane flakes found in the literature. Along with the high purity of the crystallites, a thermal stability of the resistance at 280 °C makes methyl-terminated germanane suitable for complementary metal oxide semiconductor back-end-of-line processes.
Keywords: germanane; hydration; methylation; resistivity; thermal robustness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Elias D.C., Nair R.R., Mohiuddin T.M.G., Morozov S.V., Blake P., Halsall M.P., Ferrari A.C., Boukhvalov D.W., Katsnelson M.I., Geim A.K., et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science. 2009;323:610–613. doi: 10.1126/science.1167130. - DOI - PubMed
-
- Sofo J.O., Chaudhari A.S., Barber G.D. Graphane: A two-dimensional hydrocarbon. Phys. Rev. B. 2007;75:153401. doi: 10.1103/PhysRevB.75.153401. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

