Development of a Bifunctional Ti-Based Gas Diffusion Electrode for ORR and OER by One- and Two-Step Pt-Ir Electrodeposition
- PMID: 35407351
- PMCID: PMC9003547
- DOI: 10.3390/nano12071233
Development of a Bifunctional Ti-Based Gas Diffusion Electrode for ORR and OER by One- and Two-Step Pt-Ir Electrodeposition
Abstract
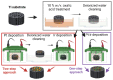
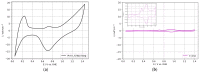
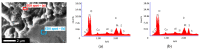
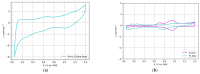
The present paper presents one- and two-step approaches for electrochemical Pt and Ir deposition on a porous Ti-substrate to obtain a bifunctional oxygen electrode. Surface pre-treatment of the fiber-based Ti-substrate with oxalic acid provides an alternative to plasma treatment for partially stripping TiO2 from the electrode surface and roughening the topography. Electrochemical catalyst deposition performed directly onto the pretreated Ti-substrates bypasses unnecessary preparation and processing of catalyst support structures. A single Pt constant potential deposition (CPD), directly followed by pulsed electrodeposition (PED), created nanosized noble agglomerates. Subsequently, Ir was deposited via PED onto the Pt sub-structure to obtain a successively deposited PtIr catalyst layer. For the co-deposition of PtIr, a binary PtIr-alloy electrolyte was used applying PED. Micrographically, areal micro- and nano-scaled Pt sub-structure were observed, supplemented by homogenously distributed, nanosized Ir agglomerates for the successive PtIr deposition. In contrast, the PtIr co-deposition led to spherical, nanosized PtIr agglomerates. The electrochemical ORR and OER activity showed increased hydrogen desorption peaks for the Pt-deposited substrate, as well as broadening and flattening of the hydrogen desorption peaks for PtIr deposited substrates. The anodic kinetic parameters for the prepared electrodes were found to be higher than those of a polished Ir-disc.
Keywords: Ti-substrate; bifunctional electrode; co- and successive Pt-Ir electrodeposition; electrolysis; fuel cell; micro- and nanostructure; ready to use electrode; unitized regenerative fuel cell; wet-chemical etching.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- United Nations . Paris Agreement. United Nations; Paris, France: 2015.
-
- Navigant T., Sach K., Jörling B., Lotz M., Jakob H., Schult D. Klimaschutz in Zahlen: Fakten, Trends und Impulse Deutscher Klimapolitik. Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit; Berlin, Germany: 2020.
-
- Europäische Kommission . Förderung Einer Klimaneutralen Wirtschaft: Kommission Legt Pläne für das Energiesystem der Zukunft und Sauberen Wasserstoff Vor. European Commission; Brussels, Belgium: 2020.
-
- Dawood F., Anda M., Shafiullah G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy. 2020;45:3847–3869. doi: 10.1016/j.ijhydene.2019.12.059. - DOI
-
- Sazali N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy. 2020;45:18753–18771. doi: 10.1016/j.ijhydene.2020.05.021. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

