Radiomics-Based Image Phenotyping of Kidney Apparent Diffusion Coefficient Maps: Preliminary Feasibility & Efficacy
- PMID: 35407587
- PMCID: PMC8999417
- DOI: 10.3390/jcm11071972
Radiomics-Based Image Phenotyping of Kidney Apparent Diffusion Coefficient Maps: Preliminary Feasibility & Efficacy
Abstract
Given the central role of interstitial fibrosis in disease progression in chronic kidney disease (CKD), a role for diffusion-weighted MRI has been pursued. We evaluated the feasibility and preliminary efficacy of using radiomic features to phenotype apparent diffusion coefficient (ADC) maps and hence to the clinical classification(s) of the participants. The study involved 40 individuals (10 healthy and 30 with CKD (eGFR < 60 mL/min/1.73 m2)). Machine learning methods, such as hierarchical clustering and logistic regression, were used. Clustering resulted in the identification of two clusters, one including all individuals with CKD (n = 17), while the second one included all the healthy volunteers (n = 10) and the remaining individuals with CKD (n = 13), resulting in 100% specificity. Logistic regression identified five radiomic features to classify participants as with CKD vs. healthy volunteers, with a sensitivity and specificity of 93% and 70%, respectively, and an AUC of 0.95. Similarly, four radiomic features were able to classify participants as rapid vs. non-rapid CKD progressors among the 30 individuals with CKD, with a sensitivity and specificity of 71% and 43%, respectively, and an AUC of 0.75. These promising preliminary data should support future studies with larger numbers of participants with varied disease severity and etiologies to improve performance.
Keywords: ADC; CKD; MRI; diffusion-weighted imaging; kidney; radiomic.
Conflict of interest statement
A.S. reports personal fees from Horizon Therapeutics, PLC, CVS Caremark, AstraZeneca, Bayer, and Tate & Latham (medicolegal consulting). All other authors declare no conflict of interest.
Figures
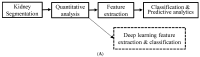






References
-
- CDC . Chronic Kidney Disease in the United States. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2021.
-
- Levey A.S., Eckardt K.-U., Dorman N.M., Christiansen S.L., Hoorn E.J., Ingelfinger J.R., Inker L.A., Levin A., Mehrotra R., Palevsky P.M., et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97:1117–1129. doi: 10.1016/j.kint.2020.02.010. - DOI - PubMed
-
- Srivastava A., Palsson R., Kaze A.D., Chen M.E., Palacios P., Sabbisetti V., Betensky R.A., Steinman T.I., Thadhani R.I., McMahon G.M., et al. The Prognostic Value of Histopathologic Lesions in Native Kidney Biopsy Specimens: Results from the Boston Kidney Biopsy Cohort Study. J. Am. Soc. Nephrol. 2018;29:2213–2224. doi: 10.1681/ASN.2017121260. - DOI - PMC - PubMed
-
- Caroli A., Schneider M., Friedli I., Ljimani A., De Seigneux S., Boor P., Gullapudi L., Kazmi I., Mendichovszky I.A., Notohamiprodjo M., et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: A systematic review and statement paper. Nephrol. Dial. Transplant. 2018;33((Suppl. 2)):ii29–ii40. doi: 10.1093/ndt/gfy163. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

