Highly Photostable Carbon Dots from Citric Acid for Bioimaging
- PMID: 35407731
- PMCID: PMC9000082
- DOI: 10.3390/ma15072395
Highly Photostable Carbon Dots from Citric Acid for Bioimaging
Abstract
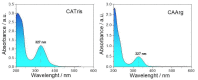
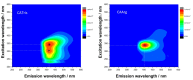
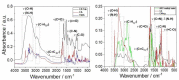
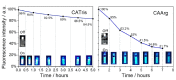
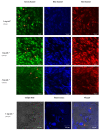
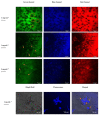
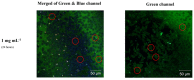
Bioimaging supported by nanoparticles requires low cost, highly emissive and photostable systems with low cytotoxicity. Carbon dots (C-dots) offer a possible solution, even if controlling their properties is not always straightforward, not to mention their potentially simple synthesis and the fact that they do not exhibit long-term photostability in general. In the present work, we synthesized two C-dots starting from citric acid and tris (hydroxymethyl)-aminomethane (tris) or arginine methyl ester dihydrochloride. Cellular uptake and bioimaging were tested in vitro using murine neuroblastoma and ovine fibroblast cells. The C-dots are highly biocompatible, and after 24 h of incubation with the cells, 100% viability was still observed. Furthermore, the C-dots synthesized using tris have an average dimension of 2 nm, a quantum yield of 37%, high photostability and a zeta potential (ζ) around -12 mV. These properties favor cellular uptake without damaging cells and allow for very effective bioimaging.
Keywords: bioimaging; carbon dots; cytotoxicity; photoluminescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Li H., Yan X., Kong D., Jin R., Sun C., Du D., Lin Y., Lu G. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz. 2019;5:218–234. doi: 10.1039/C9NH00476A. - DOI
-
- Zuo P., Lu X., Sun Z., Guo Y., He H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Mikrochim. Acta. 2015;183:519–542. doi: 10.1007/s00604-015-1705-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

