Design of New Concept of Knitted Hernia Implant
- PMID: 35408005
- PMCID: PMC9000569
- DOI: 10.3390/ma15072671
Design of New Concept of Knitted Hernia Implant
Abstract
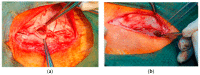
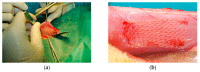
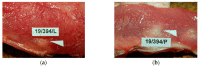
A knitted implant, unilaterally modified with plasma-assisted chemical-vapor deposition (PACVD), and with a nano-layer of fluorine derivative supplementation, for reducing the risk of complications related to adhesions, and the formation of a thick postoperative scar was prepared. The biological evaluation of designed or modified medical devices is the main aspect of preclinical research. If such studies use a medical device with prolonged contact with connective tissue (more than 30 days), biocompatibility studies require a safety assessment in terms of toxicity in vitro and in vivo, allergenicity, irritation, and cancerogenicity, reproductive and developmental toxicity. The ultimate aspect of biological evaluation is biofunctionality, and evaluation of the local tissue response after implantation, resulting in the determination of all aspects of local biocompatibility with the implemented synthetic material. The implantation of PACVD-modified materials in muscle allows us to estimate the local irritation effect on the connective tissue, determining the risk of scar formation, whereas implantation of the above-mentioned knitted fabric into the abdominal wall, assists with evaluating the risk of fistula formation-the main post-surgical complications. The research aimed to evaluate the local reaction of the soft tissues after the implantation of the knitted implants modified with PACVD of the fluoropolymer in the nanostuctural form. The local effect that occurred during the implantation of the designed implants was quantitatively and qualitatively evaluated when PACVD unmodified (reference), and modified medical devices were implanted in the abdominal cavity (intra-abdominal position) for 12 or into the muscles for 56 weeks. The comparative semi-quantitative histological assessment included the severity of inflammatory cells (multinucleated cells, lymphocytes, plasma cells, macrophages, giant cells) and the tissue response (necrosis, neovascularization, fibrosis, and fat infiltration) on a five-point scale. The knitted implants modified by PACVD did not indicate cumulative tissue response when they were implanted in the muscle and intra-abdominally with direct contact with the viscera. They reduced local tissue reaction (score -2.71 after 56 weeks of the implantation) and internal organ adhesion (irritation score -2.01 and adhesion susceptibility -0.3 after 12 weeks of the implantation) compared with the reference (unmodified by PACVD) knitted implant, which had an identical structure and was made of the same source.
Keywords: PACVD; hernia knitted implants; irritation; post-implantation effect; viscera adhesion prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Bendavid R., Abrahamson J., Arregui M.E., Flament J.B., Phillips E.H. Abdominal Wall Hernias: Principles And Management. Springer; Cham, Switzerland: 2018. pp. 39–192.
Grants and funding
LinkOut - more resources
Full Text Sources

