Tuning the Sensing Properties of N and S Co-Doped Carbon Dots for Colorimetric Detection of Copper and Cobalt in Water
- PMID: 35408102
- PMCID: PMC9003535
- DOI: 10.3390/s22072487
Tuning the Sensing Properties of N and S Co-Doped Carbon Dots for Colorimetric Detection of Copper and Cobalt in Water
Abstract
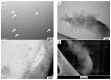
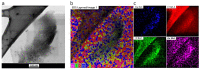
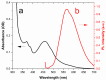
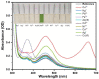
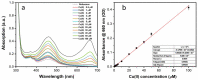
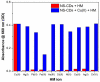
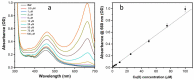
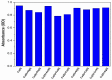
In this study, nitrogen and sulfur co-doped carbon dots (NS-CDs) were investigated for the detection of heavy metals in water through absorption-based colorimetric response. NS-CDs were synthesized by a simple one-pot hydrothermal method and characterized by TEM, STEM-coupled with energy dispersive X-ray analysis, NMR, and IR spectroscopy. Addition of Cu(II) ions to NS-CD aqueous solutions gave origin to a distinct absorption band at 660 nm which was attributed to the formation of cuprammonium complexes through coordination with amino functional groups of NS-CDs. Absorbance increased linearly with Cu(II) concentration in the range 1-100 µM and enabled a limit of detection of 200 nM. No response was observed with the other tested metals, including Fe(III) which, however, appreciably decreased sensitivity to copper. Increase of pH of the NS-CD solution up to 9.5 greatly reduced this interference effect and enhanced the response to Cu(II), thus confirming the different nature of the two interactions. In addition, a concurrent response to Co(II) appeared in a different spectral region, thus suggesting the possibility of dual-species multiple sensitivity. The present method neither requires any other reagents nor any previous assay treatment and thus can be a promising candidate for low-cost monitoring of copper onsite and by unskilled personnel.
Keywords: N and S co-doped carbon dots; colorimetry; divalent copper; optical sensing; water quality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Colorimetric and Fluorescent Sensing of Copper Ions in Water through o-Phenylenediamine-Derived Carbon Dots.Sensors (Basel). 2023 Mar 10;23(6):3029. doi: 10.3390/s23063029. Sensors (Basel). 2023. PMID: 36991739 Free PMC article.
-
Efficient dual-mode colorimetric/fluorometric sensor for the detection of copper ions and vitamin C based on pH-sensitive amino-terminated nitrogen-doped carbon quantum dots: effect of reactive oxygen species and antioxidants.Anal Bioanal Chem. 2019 May;411(12):2619-2633. doi: 10.1007/s00216-019-01710-8. Epub 2019 Mar 23. Anal Bioanal Chem. 2019. PMID: 30903223
-
Nitrogen-Doped Carbon Dots Prepared via Microchannel Method for Visual Detection of Copper Ions.Luminescence. 2025 Feb;40(2):e70113. doi: 10.1002/bio.70113. Luminescence. 2025. PMID: 39887639
-
Nitrogen and sulfur co-doped photoluminescent carbon dots for highly selective and sensitive detection of Ag+ and Hg2+ ions in aqueous media: Applications in bioimaging and real sample analysis.Environ Res. 2023 Jul 1;228:115898. doi: 10.1016/j.envres.2023.115898. Epub 2023 Apr 11. Environ Res. 2023. PMID: 37054837
-
Nitrogen-Doped Carbon Quantum Dots from Poly(ethyleneimine) for Optical Dual-Mode Determination of Cu2+ and l-Cysteine and Their Logic Gate Operation.ACS Appl Mater Interfaces. 2020 Oct 21;12(42):47245-47255. doi: 10.1021/acsami.0c12750. Epub 2020 Oct 6. ACS Appl Mater Interfaces. 2020. PMID: 32955238
Cited by
-
Colorimetric and Fluorescent Sensing of Copper Ions in Water through o-Phenylenediamine-Derived Carbon Dots.Sensors (Basel). 2023 Mar 10;23(6):3029. doi: 10.3390/s23063029. Sensors (Basel). 2023. PMID: 36991739 Free PMC article.
-
Potential Application of Carbon Dots for Sustainable Agriculture: Current Challenges and Future Prospects.J Fluoresc. 2025 Sep 1. doi: 10.1007/s10895-025-04508-3. Online ahead of print. J Fluoresc. 2025. PMID: 40889005 Review.
-
Optical Chemical Sensors: Design and Applications.Sensors (Basel). 2023 Jun 2;23(11):5284. doi: 10.3390/s23115284. Sensors (Basel). 2023. PMID: 37300010 Free PMC article.
-
A Polycaprolactone-Capped ZnO Quantum Dots-Based Fluorometric Sensor for the Detection of Fe3+ Ions in Seawater.J Fluoresc. 2024 Jul;34(4):1643-1654. doi: 10.1007/s10895-023-03394-x. Epub 2023 Aug 17. J Fluoresc. 2024. PMID: 37589936
-
Gamma-Ray-Induced Structural Transformation of GQDs towards the Improvement of Their Optical Properties, Monitoring of Selected Toxic Compounds, and Photo-Induced Effects on Bacterial Strains.Nanomaterials (Basel). 2022 Aug 7;12(15):2714. doi: 10.3390/nano12152714. Nanomaterials (Basel). 2022. PMID: 35957147 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

