Recent Advances in the Synthesis of Isoquinoline-Fused Benzimidazoles
- PMID: 35408460
- PMCID: PMC9000694
- DOI: 10.3390/molecules27072062
Recent Advances in the Synthesis of Isoquinoline-Fused Benzimidazoles
Abstract
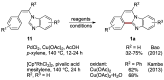
This review includes recent developments in the synthesis of benzo[4,5]imidazo[2,1-a]isoquinolines with particular attention given to categorizing protocols based on the structural features of the ring architecture and crystallographically characterized reaction products.
Keywords: benzimidazo[2,1-a]isoquinoline; benzimidazole; benzo[4,5]imidazo[2,1-a]isoquinoline; isoquinoline; nitrogen heterocycles.
Conflict of interest statement
The author declares no conflict of interest.
Figures





















References
-
- Sun Q., LaVoie E.J. Synthesis of Benzimidazo[2,1-a]isoquinolines and 5,6-Dihydrobenzimidazo[2,1-a]isoquinolines. Heterocycles. 1996;43:737–743. doi: 10.3987/com-95-7338. - DOI
-
- Deady L.W., Rodemann T., Finlay G.J., Baguley B.C., Denny W.A. Synthesis and cytotoxic activity of carboxamide derivatives of benzimidazo[2,1-a]isoquinoline and pyrido[3’,2’:4,5]imidazo[2,1-a]isoquinoline. [(accessed on 16 February 2022)];Anti-Cancer Drug Des. 2000 15:339–346. Available online: https://www.ingentaconnect.com/contentone/cog/antcan/2000/00000015/00000.... - PubMed
-
- Garrett C.E., Prasad K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004;346:889–900. doi: 10.1002/adsc.200404071. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

