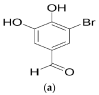
5-Bromo-3,4-dihydroxybenzaldehyde Promotes Hair Growth through Activation of Wnt/β-Catenin and Autophagy Pathways and Inhibition of TGF-β Pathways in Dermal Papilla Cells
- PMID: 35408575
- PMCID: PMC9000556
- DOI: 10.3390/molecules27072176
5-Bromo-3,4-dihydroxybenzaldehyde Promotes Hair Growth through Activation of Wnt/β-Catenin and Autophagy Pathways and Inhibition of TGF-β Pathways in Dermal Papilla Cells
Abstract
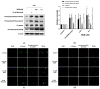
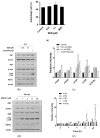
Various studies addressing the increasing problem of hair loss, using natural products with few side effects, have been conducted. 5-bromo-3,4-dihydroxybenzaldehyde (BDB) exhibited anti-inflammatory effects in mouse models of atopic dermatitis and inhibited UVB-induced oxidative stress in keratinocytes. Here, we investigated its stimulating effect and the underlying mechanism of action on hair growth using rat vibrissa follicles and dermal papilla cells (DPCs), required for the regulation of hair cycle and length. BDB increased the length of hair fibers in rat vibrissa follicles and the proliferation of DPCs, along with causing changes in the levels of cell cycle-related proteins. We investigated whether BDB could trigger anagen-activating signaling pathways, such as the Wnt/β-catenin pathway and autophagy in DPCs. BDB induces activation of the Wnt/β-catenin pathway through the phosphorylation of GSG3β and β-catenin. BDB increased the levels of autophagic vacuoles and autophagy regulatory proteins Atg7, Atg5, Atg16L, and LC3B. We also investigated whether BDB inhibits the TGF-β pathway, which promotes transition to the catagen phase. BDB inhibited the phosphorylation of Smad2 induced by TGF-β1. Thus, BDB can promote hair growth by modulating anagen signaling by activating Wnt/β-catenin and autophagy pathways and inhibiting the TGF-β pathway in DPCs.
Keywords: 5-bromo-3,4-dihydroxybenzaldehyde; TGF-β; Wnt/β-catenin; autophagy; dermal papilla cells; hair growth; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

