Synthesis and Hemostatic Activity of New Amide Derivatives
- PMID: 35408669
- PMCID: PMC9000710
- DOI: 10.3390/molecules27072271
Synthesis and Hemostatic Activity of New Amide Derivatives
Abstract
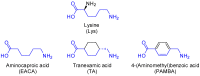
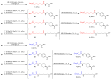
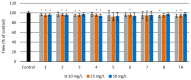
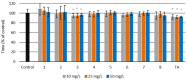
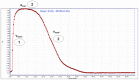
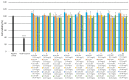
Eight dipeptides containing antifibrinolytic agents (tranexamic acid, aminocaproic acid, 4-(aminomethyl)benzoic acid, and glycine-natural amino acids) were synthesized in a three-step process with good or very good yields. DMT/NMM/TsO- (4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium toluene-4-sulfonate) was used as a coupling reagent. Hemolysis tests were used to study the effects of the dipeptides on blood components. Blood plasma clotting tests were used to examine their effects on thrombin time (TT), prothrombin time (PT), and the activated partial thromboplastin time (aPTT). The level of hemolysis did not exceed 1%. In clotting tests, TT, PT, and aPTT did not differentiate any of the compounds. The prothrombin times for all amides 1-8 were similar. The obtained results in the presence of amides 1-4 and 8 were slightly lower than for the other compounds and the positive control, and they were similar to the results obtained for TA. In the case of amide 3, a significantly decreased aPTT was observed. The aPTTs observed for plasma treated with amide 3 and TA were comparable. In the case of amide 6 and 8, TT values significantly lower than for the other compounds were found. The clot formation and fibrinolysis (CFF) assay was used to assess the influence of the dipeptides on the blood plasma coagulation cascade and the fibrinolytic efficiency of the blood plasma. In the clot formation and fibrinolysis assay, amides 5 and 7 were among the most active compounds. The cytotoxicity and genotoxicity of the synthesized dipeptides were evaluated on the monocyte/macrophage peripheral blood cell line. The dipeptides did not cause hemolysis at any concentrations. They exhibited no significant cytotoxic effect on SC cells and did not induce significant DNA damage.
Keywords: 4-(aminomethyl)benzoic acid; aminocaproic acid; antifibrinolytic agents; hemostasis; lysine analogs; peptides; tranexamic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

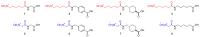





Similar articles
-
Clot Waveform Analysis for Hemostatic Abnormalities.Ann Lab Med. 2023 Nov 1;43(6):531-538. doi: 10.3343/alm.2023.43.6.531. Epub 2023 Jun 30. Ann Lab Med. 2023. PMID: 37387486 Free PMC article. Review.
-
Synthesis of Isothiocyanates Using DMT/NMM/TsO- as a New Desulfurization Reagent.Molecules. 2021 May 6;26(9):2740. doi: 10.3390/molecules26092740. Molecules. 2021. PMID: 34066597 Free PMC article.
-
Tantalum-containing mesoporous bioactive glass powder for hemostasis.J Biomater Appl. 2021 Mar;35(8):924-932. doi: 10.1177/0885328220965150. Epub 2020 Oct 15. J Biomater Appl. 2021. PMID: 33059517
-
A new anti-fibrinolytic hemostatic compound 8-O-acetyl shanzhiside methylester extracted from Lamiophlomis rotata.J Ethnopharmacol. 2016 Jul 1;187:232-8. doi: 10.1016/j.jep.2016.04.016. Epub 2016 Apr 13. J Ethnopharmacol. 2016. PMID: 27085939
-
Standardization and clinical utility of thrombin-generation assays.Semin Thromb Hemost. 2008 Oct;34(7):670-82. doi: 10.1055/s-0028-1104546. Epub 2008 Dec 15. Semin Thromb Hemost. 2008. PMID: 19085768 Review.
Cited by
-
Efficacy and safety of tranexamic acid for patients with intertrochanteric fractures treated with intramedullary fixation: A systematic review and meta-analysis of current evidence in randomized controlled trials.Front Pharmacol. 2022 Sep 19;13:945971. doi: 10.3389/fphar.2022.945971. eCollection 2022. Front Pharmacol. 2022. PMID: 36199695 Free PMC article.
-
Tranexamic acid may benefit patients with preexisting thromboembolic risk undergoing total joint arthroplasty: a systematic review and meta-analysis.EFORT Open Rev. 2024 Jun 3;9(6):467-478. doi: 10.1530/EOR-23-0140. EFORT Open Rev. 2024. PMID: 38828967 Free PMC article.
-
Anti-inflammatory effect of multi-dose tranexamic acid in hip and knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials.Inflammopharmacology. 2025 Mar;33(3):917-928. doi: 10.1007/s10787-025-01679-0. Epub 2025 Feb 24. Inflammopharmacology. 2025. PMID: 39992591
-
Biocompatibility of Phosphorus Dendrimers and Their Antibacterial Properties as Potential Agents for Supporting Wound Healing.Mol Pharm. 2025 Feb 3;22(2):927-939. doi: 10.1021/acs.molpharmaceut.4c01156. Epub 2025 Jan 11. Mol Pharm. 2025. PMID: 39797813 Free PMC article.
-
Efficacy and safety of single- and double-dose intravenous tranexamic acid in hip and knee arthroplasty: a systematic review and meta-analysis.J Orthop Surg Res. 2023 Aug 10;18(1):593. doi: 10.1186/s13018-023-03929-9. J Orthop Surg Res. 2023. PMID: 37563702 Free PMC article.
References
-
- Pharmacia & Upjohn . Cyklokapron. ABPI Compendium of Data Sheets and Summaries of Product Characteristics 1998–1999. Datapharm Publications Ltd.; London, UK: 2010.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

