Bisphenol A Impairs Lipid Remodeling Accompanying Cell Differentiation in the Oligodendroglial Cell Line Oli-Neu
- PMID: 35408676
- PMCID: PMC9000593
- DOI: 10.3390/molecules27072274
Bisphenol A Impairs Lipid Remodeling Accompanying Cell Differentiation in the Oligodendroglial Cell Line Oli-Neu
Abstract
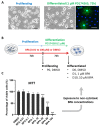
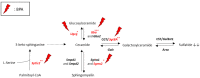
In the central nervous system, the process of myelination involves oligodendrocytes that wrap myelin around axons. Myelin sheaths are mainly composed of lipids and ensure efficient conduction of action potentials. Oligodendrocyte differentiation is an essential preliminary step to myelination which, in turn, is a key event of neurodevelopment. Bisphenol A (BPA), a ubiquitous endocrine disruptor, is suspected to disrupt this developmental process and may, thus, contribute to several neurodevelopmental disorders. In this study, we assessed the effect of BPA on oligodendrocyte differentiation through a comprehensive analysis of cell lipidome by UHPLC-HRMS. For this purpose, we exposed the oligodendroglial cell line Oli-neu to several BPA concentrations for 72 h of proliferation and another 72 h of differentiation. In unexposed cells, significant changes occurred in lipid distribution during Oli-neu differentiation, including an increase in characteristic myelin lipids, sulfatides, and ethanolamine plasmalogens, and a marked remodeling of phospholipid subclasses and fatty acid contents. Moreover, BPA induced a decrease in sulfatide and phosphatidylinositol plasmalogen contents and modified monounsaturated/polyunsaturated fatty acid relative contents in phospholipids. These effects counteracted the lipid remodeling accompanying differentiation and were confirmed by gene expression changes. Altogether, our results suggest that BPA disrupts lipid remodeling accompanying early oligodendrocyte differentiation.
Keywords: bisphenol A; differentiation; lipidomics; oligodendrocyte.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- WHO WHO|State of the Science of Endocrine Disrupting Chemicals. 2012. [(accessed on 30 March 2022)]. Available online: https://www.who.int/publications/i/item/state-of-the-science-of-endocrin....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

