The Role of mTOR and eIF Signaling in Benign Endometrial Diseases
- PMID: 35408777
- PMCID: PMC8998789
- DOI: 10.3390/ijms23073416
The Role of mTOR and eIF Signaling in Benign Endometrial Diseases
Abstract
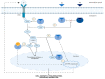
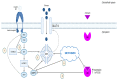
Adenomyosis, endometriosis, endometritis, and typical endometrial hyperplasia are common non-cancerous diseases of the endometrium that afflict many women with life-impacting consequences. The mammalian target of the rapamycin (mTOR) pathway interacts with estrogen signaling and is known to be dysregulated in endometrial cancer. Based on this knowledge, we attempt to investigate the role of mTOR signaling in benign endometrial diseases while focusing on how the interplay between mTOR and eukaryotic translation initiation factors (eIFs) affects their development. In fact, mTOR overactivity is apparent in adenomyosis, endometriosis, and typical endometrial hyperplasia, where it promotes endometrial cell proliferation and invasiveness. Recent data show aberrant expression of various components of the mTOR pathway in both eutopic and ectopic endometrium of patients with adenomyosis or endometriosis and in hyperplastic endometrium as well. Moreover, studies on endometritis show that derangement of mTOR signaling is linked to the establishment of endometrial dysfunction caused by chronic inflammation. This review shows that inhibition of the mTOR pathway has a promising therapeutic effect in benign endometrial conditions, concluding that mTOR signaling dysregulation plays a critical part in their pathogenesis.
Keywords: adenomyosis; eIFs; endometriosis; endometritis; mTOR signaling; typical endometrial hyperplasia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

