Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes
- PMID: 35408834
- PMCID: PMC8998825
- DOI: 10.3390/ijms23073474
Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes
Abstract
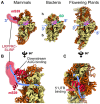
Mitochondria are key organelles that combine features inherited from their bacterial endosymbiotic ancestor with traits that arose during eukaryote evolution. These energy producing organelles have retained a genome and fully functional gene expression machineries including specific ribosomes. Recent advances in cryo-electron microscopy have enabled the characterization of a fast-growing number of the low abundant membrane-bound mitochondrial ribosomes. Surprisingly, mitoribosomes were found to be extremely diverse both in terms of structure and composition. Still, all of them drastically increased their number of ribosomal proteins. Interestingly, among the more than 130 novel ribosomal proteins identified to date in mitochondria, most of them are composed of a-helices. Many of them belong to the nuclear encoded super family of helical repeat proteins. Here we review the diversity of functions and the mode of action held by the novel mitoribosome proteins and discuss why these proteins that share similar helical folds were independently recruited by mitoribosomes during evolution in independent eukaryote clades.
Keywords: helical repeat proteins; mitochondrial gene expression; pentatricopeptide repeat proteins; ribosomes; single particle cryo-EM; translation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Striking Diversity of Mitochondria-Specific Translation Processes across Eukaryotes.Trends Biochem Sci. 2020 Feb;45(2):149-162. doi: 10.1016/j.tibs.2019.10.004. Epub 2019 Nov 25. Trends Biochem Sci. 2020. PMID: 31780199 Review.
-
Specificities of the plant mitochondrial translation apparatus.Mitochondrion. 2020 Jul;53:30-37. doi: 10.1016/j.mito.2020.04.008. Epub 2020 Apr 22. Mitochondrion. 2020. PMID: 32334144 Review.
-
Application of Cryo-EM for Visualization of Mitoribosomes.Methods Mol Biol. 2021;2192:197-210. doi: 10.1007/978-1-0716-0834-0_15. Methods Mol Biol. 2021. PMID: 33230775
-
Cryo-EM structure of the RNA-rich plant mitochondrial ribosome.Nat Plants. 2020 Apr;6(4):377-383. doi: 10.1038/s41477-020-0631-5. Epub 2020 Apr 6. Nat Plants. 2020. PMID: 32251374
-
Small is big in Arabidopsis mitochondrial ribosome.Nat Plants. 2019 Jan;5(1):106-117. doi: 10.1038/s41477-018-0339-y. Epub 2019 Jan 9. Nat Plants. 2019. PMID: 30626926
Cited by
-
Crucial role and conservation of the three [2Fe-2S] clusters in the human mitochondrial ribosome.J Biol Chem. 2025 Feb;301(2):108087. doi: 10.1016/j.jbc.2024.108087. Epub 2024 Dec 13. J Biol Chem. 2025. PMID: 39675708 Free PMC article.
-
Miniature RNAs are embedded in an exceptionally protein-rich mitoribosome via an elaborate assembly pathway.Nucleic Acids Res. 2023 Jul 7;51(12):6443-6460. doi: 10.1093/nar/gkad422. Nucleic Acids Res. 2023. PMID: 37207340 Free PMC article.
-
Research Progress in RNA-Binding Proteins.Int J Mol Sci. 2022 Dec 21;24(1):58. doi: 10.3390/ijms24010058. Int J Mol Sci. 2022. PMID: 36613501 Free PMC article.
-
Mitochondrial Neurodegenerative Diseases: Three Mitochondrial Ribosomal Proteins as Intermediate Stage in the Pathway That Associates Damaged Genes with Alzheimer's and Parkinson's.Biology (Basel). 2023 Jul 8;12(7):972. doi: 10.3390/biology12070972. Biology (Basel). 2023. PMID: 37508402 Free PMC article. Review.
-
Coordinating mitochondrial translation with assembly of the OXPHOS complexes.Hum Mol Genet. 2024 May 22;33(R1):R47-R52. doi: 10.1093/hmg/ddae025. Hum Mol Genet. 2024. PMID: 38779773 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

