An Updated Model of the Divisome: Regulation of the Septal Peptidoglycan Synthesis Machinery by the Divisome
- PMID: 35408901
- PMCID: PMC8998562
- DOI: 10.3390/ijms23073537
An Updated Model of the Divisome: Regulation of the Septal Peptidoglycan Synthesis Machinery by the Divisome
Abstract
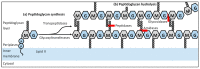
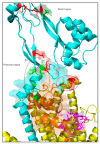
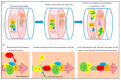
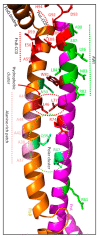
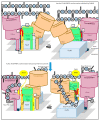
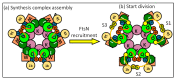
The synthesis of a peptidoglycan septum is a fundamental part of bacterial fission and is driven by a multiprotein dynamic complex called the divisome. FtsW and FtsI are essential proteins that synthesize the peptidoglycan septum and are controlled by the regulatory FtsBLQ subcomplex and the activator FtsN. However, their mode of regulation has not yet been uncovered in detail. Understanding this process in detail may enable the development of new compounds to combat the rise in antibiotic resistance. In this review, recent data on the regulation of septal peptidoglycan synthesis is summarized and discussed. Based on structural models and the collected data, multiple putative interactions within FtsWI and with regulators are uncovered. This elaborates on and supports an earlier proposed model that describes active and inactive conformations of the septal peptidoglycan synthesis complex that are stabilized by these interactions. Furthermore, a new model on the spatial organization of the newly synthesized peptidoglycan and the synthesis complex is presented. Overall, the updated model proposes a balance between several allosteric interactions that determine the state of septal peptidoglycan synthesis.
Keywords: FtsBLQ; FtsEX; FtsN; FtsWI; FtsZ; divisome; interactions; peptidoglycan hydrolases; regulation; septal peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
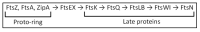











References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

