Polyploidy as a Fundamental Phenomenon in Evolution, Development, Adaptation and Diseases
- PMID: 35408902
- PMCID: PMC8998937
- DOI: 10.3390/ijms23073542
Polyploidy as a Fundamental Phenomenon in Evolution, Development, Adaptation and Diseases
Abstract
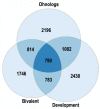
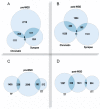
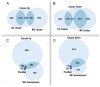
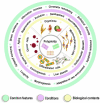
DNA replication during cell proliferation is 'vertical' copying, which reproduces an initial amount of genetic information. Polyploidy, which results from whole-genome duplication, is a fundamental complement to vertical copying. Both organismal and cell polyploidy can emerge via premature cell cycle exit or via cell-cell fusion, the latter giving rise to polyploid hybrid organisms and epigenetic hybrids of somatic cells. Polyploidy-related increase in biological plasticity, adaptation, and stress resistance manifests in evolution, development, regeneration, aging, oncogenesis, and cardiovascular diseases. Despite the prevalence in nature and importance for medicine, agri- and aquaculture, biological processes and epigenetic mechanisms underlying these fundamental features largely remain unknown. The evolutionarily conserved features of polyploidy include activation of transcription, response to stress, DNA damage and hypoxia, and induction of programs of morphogenesis, unicellularity, and longevity, suggesting that these common features confer adaptive plasticity, viability, and stress resistance to polyploid cells and organisms. By increasing cell viability, polyploidization can provide survival under stressful conditions where diploid cells cannot survive. However, in somatic cells it occurs at the expense of specific function, thus promoting developmental programming of adult cardiovascular diseases and increasing the risk of cancer. Notably, genes arising via evolutionary polyploidization are heavily involved in cancer and other diseases. Ploidy-related changes of gene expression presumably originate from chromatin modifications and the derepression of bivalent genes. The provided evidence elucidates the role of polyploidy in evolution, development, aging, and carcinogenesis, and may contribute to the development of new strategies for promoting regeneration and preventing cardiovascular diseases and cancer.
Keywords: adaptation; aging; biological plasticity; carcinogenesis; cardiovascular disease; complexity; developmental programming; epigenetic changes; evolutionary conserved features; polyploidy; regeneration; stress resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

