In Silico Characterisation of the Late Embryogenesis Abundant (LEA) Protein Families and Their Role in Desiccation Tolerance in Ramonda serbica Panc
- PMID: 35408906
- PMCID: PMC8998581
- DOI: 10.3390/ijms23073547
In Silico Characterisation of the Late Embryogenesis Abundant (LEA) Protein Families and Their Role in Desiccation Tolerance in Ramonda serbica Panc
Abstract
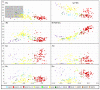
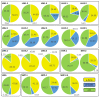
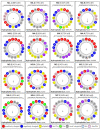
Ramonda serbica Panc. is an ancient resurrection plant able to survive a long desiccation period and recover metabolic functions upon watering. The accumulation of protective late embryogenesis abundant proteins (LEAPs) is a desiccation tolerance hallmark. To propose their role in R. serbica desiccation tolerance, we structurally characterised LEAPs and evaluated LEA gene expression levels in hydrated and desiccated leaves. By integrating de novo transcriptomics and homologues LEAP domains, 318 R. serbica LEAPs were identified and classified according to their conserved motifs and phylogeny. The in silico analysis revealed that hydrophilic LEA4 proteins exhibited an exceptionally high tendency to form amphipathic α-helices. The most abundant, atypical LEA2 group contained more hydrophobic proteins predicted to fold into the defined globular domains. Within the desiccation-upregulated LEA genes, the majority encoded highly disordered DEH1, LEA1, LEA4.2, and LEA4.3 proteins, while the greatest portion of downregulated genes encoded LEA2.3 and LEA2.5 proteins. While dehydrins might chelate metals and bind DNA under water deficit, other intrinsically disordered LEAPs might participate in forming intracellular proteinaceous condensates or adopt amphipathic α-helical conformation, enabling them to stabilise desiccation-sensitive proteins and membranes. This comprehensive LEAPs structural characterisation is essential to understanding their function and regulation during desiccation aiming at crop drought tolerance improvement.
Keywords: 3D protein structure modelling; de novo transcriptome assembly; differentially expressed gene analysis; drought; intrinsically disordered proteins; liquid–liquid phase separation; resurrection plants; secondary structure prediction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
- grant no. 6039663/Science Fund of the Republic of Serbia
- No. 451-03-68/2022-14/200042, 2022/Ministry of Education, Science and Technological Development, the Republic of Serbia
- 451-03-9/2021-14/200053, 2021/Ministry of Education, Science and Technological Development, the Republic of Serbia
- 451-03-01963/2017-09/09/Ministry of Education, Science and Technological Development, the Republic of Serbia
LinkOut - more resources
Full Text Sources

