Calcium- and Integrin-Binding Protein 2 (CIB2) in Physiology and Disease: Bright and Dark Sides
- PMID: 35408910
- PMCID: PMC8999013
- DOI: 10.3390/ijms23073552
Calcium- and Integrin-Binding Protein 2 (CIB2) in Physiology and Disease: Bright and Dark Sides
Abstract
Calcium- and integrin-binding protein 2 (CIB2) is a small EF-hand protein capable of binding Mg2+ and Ca2+ ions. While its biological function remains largely unclear, an increasing number of studies have shown that CIB2 is an essential component of the mechano-transduction machinery that operates in cochlear hair cells. Mutations in the gene encoding CIB2 have been associated with non-syndromic deafness. In addition to playing an important role in the physiology of hearing, CIB2 has been implicated in a multitude of very different processes, ranging from integrin signaling in platelets and skeletal muscle to autophagy, suggesting extensive functional plasticity. In this review, we summarize the current understanding of biochemical and biophysical properties of CIB2 and the biological roles that have been proposed for the protein in a variety of processes. We also highlight the many molecular aspects that remain unclarified and deserve further investigation.
Keywords: Usher syndrome; hearing; integrin signaling; mechanoelectrical transduction; non-syndromic deafness.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures

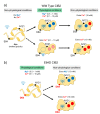
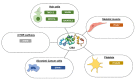
Similar articles
-
The Diverse Functions of the Calcium- and Integrin-Binding Protein Family.Int J Mol Sci. 2025 Feb 28;26(5):2223. doi: 10.3390/ijms26052223. Int J Mol Sci. 2025. PMID: 40076845 Free PMC article. Review.
-
Structural insights into calcium-dependent CIB2-TMC1 interaction in hair cell mechanotransduction.Commun Biol. 2025 Feb 25;8(1):306. doi: 10.1038/s42003-025-07761-1. Commun Biol. 2025. PMID: 40000792 Free PMC article.
-
Loss of CIB2 Causes Profound Hearing Loss and Abolishes Mechanoelectrical Transduction in Mice.Front Mol Neurosci. 2017 Dec 4;10:401. doi: 10.3389/fnmol.2017.00401. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29255404 Free PMC article.
-
Advances in genetic hearing loss: CIB2 gene.Eur Arch Otorhinolaryngol. 2017 Apr;274(4):1791-1795. doi: 10.1007/s00405-016-4330-9. Epub 2016 Oct 22. Eur Arch Otorhinolaryngol. 2017. PMID: 27771768 Free PMC article. Review.
-
Cib2 binds integrin alpha7Bbeta1D and is reduced in laminin alpha2 chain-deficient muscular dystrophy.J Biol Chem. 2008 Sep 5;283(36):24760-9. doi: 10.1074/jbc.M801166200. Epub 2008 Jul 7. J Biol Chem. 2008. PMID: 18611855 Free PMC article.
Cited by
-
Usher syndrome proteins ADGRV1 (USH2C) and CIB2 (USH1J) interact and share a common interactome containing TRiC/CCT-BBS chaperonins.Front Cell Dev Biol. 2023 Jun 22;11:1199069. doi: 10.3389/fcell.2023.1199069. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37427378 Free PMC article.
-
The Diverse Functions of the Calcium- and Integrin-Binding Protein Family.Int J Mol Sci. 2025 Feb 28;26(5):2223. doi: 10.3390/ijms26052223. Int J Mol Sci. 2025. PMID: 40076845 Free PMC article. Review.
-
Differentially Expressed Genes in Cardiomyocytes of the First Camelized Mouse Model, Nrapc.255ins78 Mouse.Genes (Basel). 2025 Jan 24;16(2):142. doi: 10.3390/genes16020142. Genes (Basel). 2025. PMID: 40004470 Free PMC article.
-
Structural insights into calcium-dependent CIB2-TMC1 interaction in hair cell mechanotransduction.Commun Biol. 2025 Feb 25;8(1):306. doi: 10.1038/s42003-025-07761-1. Commun Biol. 2025. PMID: 40000792 Free PMC article.
-
Autosomal recessive non-syndromic hearing loss genes in Pakistan during the previous three decades.J Cell Mol Med. 2024 Apr;28(8):e18119. doi: 10.1111/jcmm.18119. J Cell Mol Med. 2024. PMID: 38534090 Free PMC article. Review.
References
-
- Seki N., Hattori A., Hayashi A., Kozuma S., Ohira M., Hori T., Saito T. Structure, expression profile and chromosomal location of an isolog of DNA-PKcs interacting protein (KIP) gene. Biochim. Biophys. Acta. 1999;1444:143–147. - PubMed
-
- Riazuddin S., Belyantseva I.A., Giese A.P., Lee K., Indzhykulian A.A., Nandamuri S.P., Yousaf R., Sinha G.P., Lee S., Terrell D., et al. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet. 2012;44:1265–1271. doi: 10.1038/ng.2426. - DOI - PMC - PubMed
-
- Hager M., Bigotti M.G., Meszaros R., Carmignac V., Holmberg J., Allamand V., Akerlund M., Kalamajski S., Brancaccio A., Mayer U., et al. Cib2 binds integrin alpha7Bbeta1D and is reduced in laminin alpha2 chain-deficient muscular dystrophy. J. Biol. Chem. 2008;283:24760–24769. doi: 10.1074/jbc.M801166200. - DOI - PMC - PubMed
-
- Blazejczyk M., Sobczak A., Debowska K., Wisniewska M.B., Kirilenko A., Pikula S., Jaworski J., Kuznicki J., Wojda U. Biochemical characterization and expression analysis of a novel EF-hand Ca2+ binding protein calmyrin2 (Cib2) in brain indicates its function in NMDA receptor mediated Ca2+ signaling. Arch. Biochem. Biophys. 2009;487:66–78. doi: 10.1016/j.abb.2009.05.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

