Melatonin Positively Regulates Both Dark- and Age-Induced Leaf Senescence by Reducing ROS Accumulation and Modulating Abscisic Acid and Auxin Biosynthesis in Cucumber Plants
- PMID: 35408936
- PMCID: PMC8998517
- DOI: 10.3390/ijms23073576
Melatonin Positively Regulates Both Dark- and Age-Induced Leaf Senescence by Reducing ROS Accumulation and Modulating Abscisic Acid and Auxin Biosynthesis in Cucumber Plants
Abstract
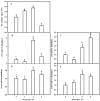
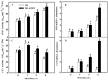
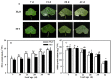
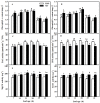
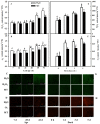
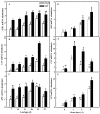
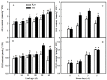
Melatonin (MT), as a signaling molecule, plays a vital role in regulating leaf senescence in plants. This study aimed to verify the antioxidant roles of MT in delaying dark- or age-induced leaf senescence of cucumber plants. The results showed that endogenous MT responds to darkness and overexpression of CsASMT, the key gene of MT synthesis, and delays leaf senescence stimulated by darkness, as manifested by significantly lower malonaldehyde (MDA) and reactive oxygen species (ROS) contents as well as higher activities and gene expression of antioxidant enzymes compared to the control. Moreover, MT suppressed both age- or dark-induced leaf senescence of cucumber, as evidenced by a decrease in senescence-related gene SAG20 and cell-death-related gene PDCD expression and ROS content and an increase in antioxidant capacity and chlorophyll biosynthesis compared with the H2O-treated seedlings. Meanwhile, the suppression of age-induced leaf senescence by melatonin was also reflected by the reduction in abscisic acid (ABA) biosynthesis and signaling pathways as well as the promotion of auxin (IAA) biosynthesis and signaling pathways in cucumber plants in the solar greenhouse. Combining the results of the two separate experiments, we demonstrated that MT acts as a powerful antioxidant to alleviate leaf senescence by activating the antioxidant system and IAA synthesis and signaling while inhibiting ABA synthesis and signaling in cucumber plants.
Keywords: abscisic acid; antioxidant system; auxin; cucumber; leaf senescence; melatonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sun J.L., Sui X.L., Huang H.Y., Wang S.H., Wei Y.X., Zhang Z.X. Low Light Stress Down-Regulated Rubisco Gene Expression and Photosynthetic Capacity During Cucumber (Cucumis sativus L.) Leaf Development. J. Integr. Agric. 2014;13:997–1007. doi: 10.1016/S2095-3119(13)60670-X. - DOI
-
- Wu H.Y., Dong F.Q., Liu L.A., Shi L., Zhang W.F., Jiang C.D. Dorsoventral variation in photosynthesis during leaf senescence probed by chlorophyll a fluorescence induction kinetics in cucumber and maize plants. Photosynthetica. 2020;58:479–487. doi: 10.32615/ps.2020.005. - DOI
-
- Betania F.Q., Yoo-Sun N., Edward H., Richard M.A. Molecular aspects of leaf senescence. Trends Plant Sci. 2000;5:278–282. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

