Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus
- PMID: 35408946
- PMCID: PMC8999085
- DOI: 10.3390/ijms23073587
Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus
Abstract
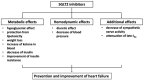
There is a close relationship between diabetes mellitus and heart failure, and diabetes is an independent risk factor for heart failure. Diabetes and heart failure are linked by not only the complication of ischemic heart disease, but also by metabolic disorders such as glucose toxicity and lipotoxicity based on insulin resistance. Cardiac dysfunction in the absence of coronary artery disease, hypertension, and valvular disease is called diabetic cardiomyopathy. Diabetes-induced hyperglycemia and hyperinsulinemia lead to capillary damage, myocardial fibrosis, and myocardial hypertrophy with mitochondrial dysfunction. Lipotoxicity with extensive fat deposits or lipid droplets is observed on cardiomyocytes. Furthermore, increased oxidative stress and inflammation cause cardiac fibrosis and hypertrophy. Treatment with a sodium glucose cotransporter 2 (SGLT2) inhibitor is currently one of the most effective treatments for heart failure associated with diabetes. However, an effective treatment for lipotoxicity of the myocardium has not yet been established, and the establishment of an effective treatment is needed in the future. This review provides an overview of heart failure in diabetic patients for the clinical practice of clinicians.
Keywords: SGLT2 inhibitor; heart failure; lipotoxicity.
Conflict of interest statement
Nakamura received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Novartis Pharma K.K., MSD K.K., and AstraZeneca plc. outside the submitted work. Miyoshi received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Novartis Pharma K.K., and MSD K.K. outside the submitted work. Dr. Masashi Yoshida received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., and Novartis Pharma K.K. outside the submitted work. Akagi received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., and Novartis Pharma K.K. outside the submitted work. Ejiri received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Novartis Pharma K.K. outside the submitted work. Ito received scholarship donation and lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Novartis Pharma K.K., MSD K.K., AstraZeneca plc., Ono Pharmaceutical Co. Ltd., Daiichi-Sankyo Pharmaceutical Co. Ltd., and Tanabe-Mitsubishi Pharmaceutical Co. Ltd., outside the submitted work. The other authors declare no conflict of interest.
Figures

References
-
- Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

