TRPV2: A Key Player in Myelination Disorders of the Central Nervous System
- PMID: 35408977
- PMCID: PMC8999035
- DOI: 10.3390/ijms23073617
TRPV2: A Key Player in Myelination Disorders of the Central Nervous System
Abstract
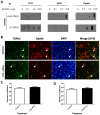
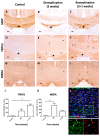
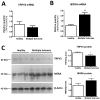
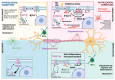
Transient potential receptor vanilloid 2 (TRPV2) is widely expressed through the nervous system and specifically found in neuronal subpopulations and some glial cells. TRPV2 is known to be sensitized by methionine oxidation, which results from inflammation. Here we aim to characterize the expression and regulation of TRPV2 in myelination pathologies, such as hypomyelination and demyelination. We validated the interaction between TRPV2 and its putative interactor Opalin, an oligodendrocyte marker, in mixed glial cultures under pro- and anti-inflammatory conditions. Then, we characterized TRPV2 time-course expression in experimental animal models of hypomyelination (jimpy mice) and de-/remyelination (cuprizone intoxication and experimental autoimmune encephalomyelitis (EAE)). TRPV2 showed upregulation associated with remyelination, inflammation in cuprizone and EAE models, and downregulation in hypomyelinated jimpy mice. TRPV2 expression was altered in human samples of multiple sclerosis (MS) patients. Additionally, we analyzed the expression of methionine sulfoxide reductase A (MSRA), an enzyme that reduces oxidated methionines in TRPV2, which we found increased in inflammatory conditions. These results suggest that TRPV2 may be a key player in myelination in accordance with the recapitulation hypothesis, and that it may become an interesting clinical target in the treatment of demyelination disorders.
Keywords: Opalin; multiple sclerosis; myelination; oxidative stress; recapitulation theory; transient potential receptor vanilloid 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fricke T.C., Echtermeyer F., Zielke J., de la Roche J., Filipovic M.R., Claverol S., Herzog C., Tominaga M., Pumroy R.A., Moiseenkova-Bell V.Y., et al. Oxidation of methionine residues activates the high-threshold heat-sensitive ion channel TRPV2. Proc. Natl. Acad. Sci. USA. 2019;116:24359–24365. doi: 10.1073/pnas.1904332116. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

