Protective Effects of Mitochondrial Uncoupling Protein 2 against Aristolochic Acid I-Induced Toxicity in HK-2 Cells
- PMID: 35409033
- PMCID: PMC8998172
- DOI: 10.3390/ijms23073674
Protective Effects of Mitochondrial Uncoupling Protein 2 against Aristolochic Acid I-Induced Toxicity in HK-2 Cells
Abstract
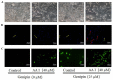
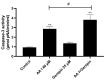
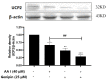
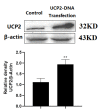
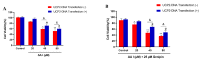
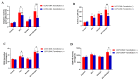
Aristolochic acid I (AA I) is one of the most abundant and toxic aristolochic acids that is reported to cause Aristolochic acid nephropathy (AAN). This paper was designed to assess whether mitochondrial Uncoupling Protein 2 (UCP2), which plays an antioxidative and antiapoptotic role, could protect human renal proximal tubular epithelial (HK-2) cells from toxicity induced by AA I. In this study, HK-2 cells were treated with different concentrations of AA I with or without UCP2 inhibitor (genipin). To upregulate the expression of UCP2 in HK-2 cells, UCP2-DNA transfection was performed. The cell viability was evaluated by colorimetric method using MTT. A series of related biological events such as Reactive Oxygen Species (ROS), Glutathione peroxidase (GSH-Px), and Malondialdehyde (MDA) were evaluated. The results showed that the cytotoxicity of AA I with genipin group was much higher than that of AA I alone. Genipin dramatically boosted oxidative stress and exacerbated AA I-induced apoptosis. Furthermore, the increased expression of UCP2 can reduce the toxicity of AA I on HK-2 cells and upregulation of UCP2 expression can reduce AA I-induced oxidative stress and apoptosis. In conclusion, UCP2 might be a potential target for alleviating AA I-induced nephrotoxicity.
Keywords: apoptosis; aristolochic acid I (AA I); nephrotoxicity; oxidative stress; uncoupling protein 2 (UCP2).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

