Proteomic Profiling of Saliva and Tears in Radiated Head and Neck Cancer Patients as Compared to Primary Sjögren's Syndrome Patients
- PMID: 35409074
- PMCID: PMC8998953
- DOI: 10.3390/ijms23073714
Proteomic Profiling of Saliva and Tears in Radiated Head and Neck Cancer Patients as Compared to Primary Sjögren's Syndrome Patients
Abstract
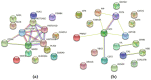
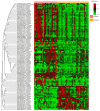
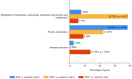
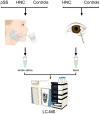
Patients with head and neck cancer (HNC) and patients with primary Sjögren's syndrome (pSS) may exhibit similar symptoms of dry mouth and dry eyes, as a result of radiotherapy (RT) or a consequence of disease progression. To identify the proteins that may serve as promising disease biomarkers, we analysed saliva and tears from 29 radiated HNC patients and 21 healthy controls, and saliva from 14 pSS patients by mass spectrometry-based proteomics. The study revealed several upregulated, and in some instances overlapping, proteins in the two patient groups. Histone H1.4 and neutrophil collagenase were upregulated in whole saliva of both patient groups, while caspase-14, histone H4, and protein S100-A9 were upregulated in HNC saliva only. In HCN tear fluid, the most highly upregulated protein was mucin-like protein 1. These overexpressed proteins in saliva and tears play central roles in inflammation, host cell injury, activation of reactive oxygen species, and tissue repair. In conclusion, the similarities and differences in overexpressed proteins detected in saliva from HNC and pSS patients may contribute to the overall understanding of the different pathophysiological mechanisms inducing dry mouth. Thus, the recurring proteins identified could possibly serve as future promising biomarkers.
Keywords: Sjögren’s syndrome; biomarkers; head-and-neck cancer; immune response; inflammation; lacrimal glands; meibomian glands; proteomics; radiotherapy; saliva; salivary glands; tear fluid; tissue healing.
Conflict of interest statement
T.P.U. is co-founder and co-owner of The Norwegian dry eye clinic and The clinic of eye health, Oslo, Norway. He has served on the global scientific advisory board for Novartis and Alcon as well as the European advisory board for Shire Pharmaceuticals.
Figures




References
-
- Pfister D.G., Spencer S., Adelstein D., Adkins D., Anzai Y., Brizel D.M., Bruce J.Y., Busse P.M., Caudell J.J., Cmelak A.J., et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020;18:873–898. doi: 10.6004/jnccn.2020.0031. - DOI - PubMed
-
- Roscher I., Falk R.S., Vos L., Clausen O.P.F., Helsing P., Gjersvik P., Robsahm T.E. Notice of Retraction and Replacement: Roscher et al. Validating 4 Staging Systems for Cutaneous Squamous Cell Carcinoma Using Population-Based Data: A Nested Case-Control Study. JAMA Dermatol. 2018;154:1488–1489. doi: 10.1001/jamadermatol.2018.4023. - DOI - PubMed
-
- Jelonek K., Krzywon A., Jablonska P., Slominska E.M., Smolenski R.T., Polanska J., Rutkowski T., Mrochem-Kwarciak J., Skladowski K., Widlak P. Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles. Metabolites. 2020;10:60. doi: 10.3390/metabo10020060. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

