Specific Attenuation of Purinergic Signaling during Bortezomib-Induced Peripheral Neuropathy In Vitro
- PMID: 35409095
- PMCID: PMC8998302
- DOI: 10.3390/ijms23073734
Specific Attenuation of Purinergic Signaling during Bortezomib-Induced Peripheral Neuropathy In Vitro
Abstract
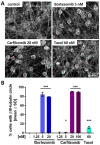
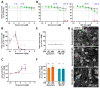
Human peripheral neuropathies are poorly understood, and the availability of experimental models limits further research. The PeriTox test uses immature dorsal root ganglia (DRG)-like neurons, derived from induced pluripotent stem cells (iPSC), to assess cell death and neurite damage. Here, we explored the suitability of matured peripheral neuron cultures for the detection of sub-cytotoxic endpoints, such as altered responses of pain-related P2X receptors. A two-step differentiation protocol, involving the transient expression of ectopic neurogenin-1 (NGN1) allowed for the generation of homogeneous cultures of sensory neurons. After >38 days of differentiation, they showed a robust response (Ca2+-signaling) to the P2X3 ligand α,β-methylene ATP. The clinical proteasome inhibitor bortezomib abolished the P2X3 signal at ≥5 nM, while 50−200 nM was required in the PeriTox test to identify neurite damage and cell death. A 24 h treatment with low nM concentrations of bortezomib led to moderate increases in resting cell intracellular Ca2+ concentration but signaling through transient receptor potential V1 (TRPV1) receptors or depolarization-triggered Ca2+ influx remained unaffected. We interpreted the specific attenuation of purinergic signaling as a functional cell stress response. A reorganization of tubulin to form dense structures around the cell somata confirmed a mild, non-cytotoxic stress triggered by low concentrations of bortezomib. The proteasome inhibitors carfilzomib, delanzomib, epoxomicin, and MG-132 showed similar stress responses. Thus, the model presented here may be used for the profiling of new proteasome inhibitors in regard to their side effect (neuropathy) potential, or for pharmacological studies on the attenuation of their neurotoxicity. P2X3 signaling proved useful as endpoint to assess potential neurotoxicants in peripheral neurons.
Keywords: bortezomib; nociceptors; peripheral nervous system diseases; proteasome inhibitors; purinergic receptor P2X3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Evaluation of tubulin polymerization and chronic inhibition of proteasome as citotoxicity mechanisms in bortezomib-induced peripheral neuropathy.Cell Cycle. 2014;13(4):612-21. doi: 10.4161/cc.27476. Epub 2013 Dec 12. Cell Cycle. 2014. PMID: 24335344
-
The Association of Neuronal Stress with Activating Transcription Factor 3 in Dorsal Root Ganglion of in vivo and in vitro Models of Bortezomib- Induced Neuropathy.Curr Cancer Drug Targets. 2019;19(1):50-64. doi: 10.2174/1568009618666181003170027. Curr Cancer Drug Targets. 2019. PMID: 30289077
-
Generation of Human Nociceptor-Enriched Sensory Neurons for the Study of Pain-Related Dysfunctions.Stem Cells Transl Med. 2022 Jul 20;11(7):727-741. doi: 10.1093/stcltm/szac031. Stem Cells Transl Med. 2022. PMID: 35689659 Free PMC article.
-
Neurotoxicity induced by antineoplastic proteasome inhibitors.Neurotoxicology. 2014 Jul;43:28-35. doi: 10.1016/j.neuro.2014.02.001. Epub 2014 Feb 10. Neurotoxicology. 2014. PMID: 24525285 Review.
-
Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy.Int J Mol Sci. 2021 Jan 17;22(2):888. doi: 10.3390/ijms22020888. Int J Mol Sci. 2021. PMID: 33477371 Free PMC article. Review.
Cited by
-
Optimization of a human induced pluripotent stem cell-derived sensory neuron model for the in vitro evaluation of taxane-induced neurotoxicity.Sci Rep. 2024 Aug 17;14(1):19075. doi: 10.1038/s41598-024-69280-z. Sci Rep. 2024. PMID: 39154055 Free PMC article.
-
Dynamic Metabolic and Transcriptional Responses of Proteasome-Inhibited Neurons.Antioxidants (Basel). 2023 Jan 10;12(1):164. doi: 10.3390/antiox12010164. Antioxidants (Basel). 2023. PMID: 36671027 Free PMC article.
-
Electrophysiological Analyses of Human Dorsal Root Ganglia and Human Induced Pluripotent Stem Cell-derived Sensory Neurons From Male and Female Donors.J Pain. 2024 Jun;25(6):104451. doi: 10.1016/j.jpain.2023.12.008. Epub 2023 Dec 27. J Pain. 2024. PMID: 38154622 Free PMC article.
-
Chemotherapy-induced peripheral neuropathy models constructed from human induced pluripotent stem cells and directly converted cells: a systematic review.Pain. 2024 Sep 1;165(9):1914-1925. doi: 10.1097/j.pain.0000000000003193. Epub 2024 Feb 21. Pain. 2024. PMID: 38381959 Free PMC article.
-
Using Human iPSC-Derived Peripheral Nervous System Disease Models for Drug Discovery.Handb Exp Pharmacol. 2023;281:191-205. doi: 10.1007/164_2023_690. Handb Exp Pharmacol. 2023. PMID: 37815594
References
-
- Csizmadia V., Raczynski A., Csizmadia E., Fedyk E.R., Rottman J., Alden C.L. Effect of an experimental proteasome inhibitor on the cytoskeleton, cytosolic protein turnover, and induction in the neuronal cells in vitro. Neurotoxicology. 2008;29:232–243. doi: 10.1016/j.neuro.2007.11.003. - DOI - PubMed
-
- Staff N.P., Podratz J.L., Grassner L., Bader M., Paz J., Knight A.M., Loprinzi C.L., Trushina E., Windebank A.J. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology. 2013;39:124–131. doi: 10.1016/j.neuro.2013.09.001. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

