Eugenol β-Amino/β-Alkoxy Alcohols with Selective Anticancer Activity
- PMID: 35409123
- PMCID: PMC8999083
- DOI: 10.3390/ijms23073759
Eugenol β-Amino/β-Alkoxy Alcohols with Selective Anticancer Activity
Abstract
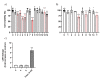
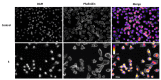
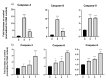
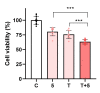
Eugenol, 4-allyl-2-methoxyphenol, is the main constituent of clove essential oil and has demonstrated relevant biological activity, namely anticancer activity. Aiming to increase this activity, we synthesized a series of eugenol β-amino alcohol and β-alkoxy alcohol derivatives, which were then tested against two human cancer cell lines, namely gastric adenocarcinoma cells (AGS) and lung adenocarcinoma cells (A549). An initial screening was performed to identify the most cytotoxic compounds. The results demonstrated that three β-amino alcohol derivatives had anticancer activity that justified subsequent studies, having been shown to trigger apoptosis. Importantly, the most potent molecules displayed no appreciable toxicity towards human noncancer cells. Structure-activity relationships show that changes in eugenol structure led to enhanced cytotoxic activity and can contribute to the future design of more potent and selective drugs.
Keywords: anticancer; apoptosis; cytotoxicity; eugenol; β-alkoxy alcohols; β-amino alcohols.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- World Health Organization Cancer. [(accessed on 11 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

