A Liquid Biopsy-Based Approach for Monitoring Treatment Response in Post-Operative Colorectal Cancer Patients
- PMID: 35409133
- PMCID: PMC8998310
- DOI: 10.3390/ijms23073774
A Liquid Biopsy-Based Approach for Monitoring Treatment Response in Post-Operative Colorectal Cancer Patients
Abstract
Monitoring the therapeutic response of colorectal cancer (CRC) patients is crucial to determine treatment strategies; therefore, we constructed a liquid biopsy-based approach for tracking tumor dynamics in non-metastatic (nmCRC) and metastatic (mCRC) patients (n = 55). Serial blood collections were performed during chemotherapy for measuring the amount and the global methylation pattern of cell-free DNA (cfDNA), the promoter methylation of SFRP2 and SDC2 genes, and the plasma homocysteine level. The average cfDNA amount was higher (p < 0.05) in nmCRC patients with recurrent cancer (30.4 ± 17.6 ng) and mCRC patients with progressive disease (PD) (44.3 ± 34.5 ng) compared to individuals with remission (13.2 ± 10.0 ng) or stable disease (12.5 ± 3.4 ng). More than 10% elevation of cfDNA from first to last sample collection was detected in all recurrent cases and 92% of PD patients, while a decrease was observed in most patients with remission. Global methylation level changes indicated a decline (75.5 ± 3.4% vs. 68.2 ± 8.4%), while the promoter methylation of SFRP2 and SDC2 and homocysteine level (10.9 ± 3.4 µmol/L vs. 13.7 ± 4.3 µmol/L) presented an increase in PD patients. In contrast, we found exact opposite changes in remission cases. Our study offers a more precise blood-based approach to monitor the treatment response to different chemotherapies than the currently used markers.
Keywords: DNA methylation; cell-free DNA; colorectal cancer; homocysteine; therapeutic response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
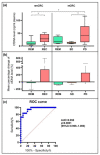
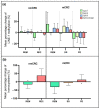

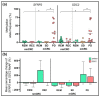
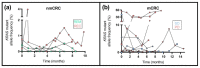
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

