In Pancreatic Adenocarcinoma Alpha-Synuclein Increases and Marks Peri-Neural Infiltration
- PMID: 35409135
- PMCID: PMC8999122
- DOI: 10.3390/ijms23073775
In Pancreatic Adenocarcinoma Alpha-Synuclein Increases and Marks Peri-Neural Infiltration
Abstract
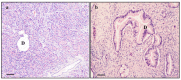
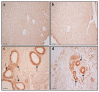
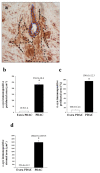
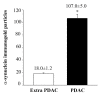
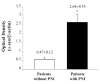
α-Synuclein (α-syn) is a protein involved in neuronal degeneration. However, the family of synucleins has recently been demonstrated to be involved in the mechanisms of oncogenesis by selectively accelerating cellular processes leading to cancer. Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human cancers, with a specifically high neurotropism. The molecular bases of this biological behavior are currently poorly understood. Here, α-synuclein was analyzed concerning the protein expression in PDAC and the potential association with PDAC neurotropism. Tumor (PDAC) and extra-tumor (extra-PDAC) samples from 20 patients affected by PDAC following pancreatic resections were collected at the General Surgery Unit, University of Pisa. All patients were affected by moderately or poorly differentiated PDAC. The amount of α-syn was compared between tumor and extra-tumor specimen (sampled from non-affected neighboring pancreatic areas) by using in situ immuno-staining with peroxidase anti-α-syn immunohistochemistry, α-syn detection by using Western blotting, and electron microscopy by using α-syn-conjugated immuno-gold particles. All the methods consistently indicate that each PDAC sample possesses a higher amount of α-syn compared with extra-PDAC tissue. Moreover, the expression of α-syn was much higher in those PDAC samples from tumors with perineural infiltration compared with tumors without perineural infiltration.
Keywords: Western blotting; electron microscopy; electron-microscopy; neuroinvasion; pancreatic ductal adenocarcinoma; ultrastructural stoichiometry; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

