FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms
- PMID: 35409166
- PMCID: PMC8998816
- DOI: 10.3390/ijms23073800
FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms
Abstract
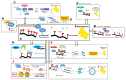
Fat mass and obesity-associated protein (FTO) is the first reported RNA N6-methyladenosine (m6A) demethylase in eukaryotic cells. m6A is considered as the most abundant mRNA internal modification, which modulates several cellular processes including alternative splicing, stability, and expression. Genome-wide association studies (GWAS) identified single-nucleotide polymorphisms (SNPs) within FTO to be associated with obesity, as well as cancer including endometrial cancer, breast cancer, pancreatic cancer, and melanoma. Since the initial classification of FTO as an m6A demethylase, various studies started to unravel a connection between FTO's demethylase activity and the susceptibility to obesity on the molecular level. FTO was found to facilitate adipogenesis, by regulating adipogenic pathways and inducing pre-adipocyte differentiation. FTO has also been investigated in tumorigenesis, where emerging studies suggest m6A and FTO levels are dysregulated in various cancers, including acute myeloid leukemia (AML), glioblastoma, cervical squamous cell carcinoma (CSCC), breast cancer, and melanoma. Here we review the molecular bases of m6A in tumorigenesis and adipogenesis while highlighting the controversial role of FTO in obesity. We provide recent findings confirming FTO's causative link to obesity and discuss novel approaches using RNA demethylase inhibitors as targeted oncotherapies. Our review aims to confirm m6A demethylation as a risk factor in obesity and provoke new research in FTO and human disorders.
Keywords: N6-methyladenosine (m6A); adipogenesis; cancer; fat mass and obesity-associated (FTO) protein; obesity; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gerken T., Girard C.A., Tung Y.-C.L., Webby C.J., Saudek V., Hewitson K.S., Yeo G.S.H., McDonough M.A., Cunliffe S., McNeill L.A., et al. The Obesity-Associated FTO Gene Encodes a 2-Oxoglutarate-Dependent Nucleic Acid Demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

