Transcriptional Regulation of RIP2 Gene by NFIB Is Associated with Cellular Immune and Inflammatory Response to APEC Infection
- PMID: 35409172
- PMCID: PMC8998712
- DOI: 10.3390/ijms23073814
Transcriptional Regulation of RIP2 Gene by NFIB Is Associated with Cellular Immune and Inflammatory Response to APEC Infection
Abstract
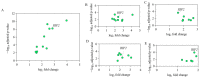
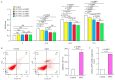
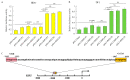
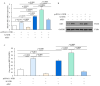
Avian pathogenic E. coli (APEC) can cause localized or systemic infection, resulting in large economic losses per year, and impact health of humans. Previous studies showed that RIP2 (receptor interacting serine/threonine kinase 2) and its signaling pathway played an important role in immune response against APEC infection. In this study, chicken HD11 cells were used as an in vitro model to investigate the function of chicken RIP2 and the transcription factor binding to the RIP2 core promoter region via gene overexpression, RNA interference, RT-qPCR, Western blotting, dual luciferase reporter assay, CHIP-PCR, CCK-8, and flow cytometry assay following APEC stimulation. Results showed that APEC stimulation promoted RIP2 expression and cells apoptosis, and inhibited cells viability. Knockdown of RIP2 significantly improved cell viability and suppressed the apoptosis of APEC-stimulated cells. Transcription factor NFIB (Nuclear factor I B) and GATA1 (globin transcription factor 1) binding site was identified in the core promoter region of RIP2 from -2300 bp to -1839 bp. However, only NFIB was confirmed to be bound to the core promoter of RIP2. Overexpression of NFIB exacerbated cell injuries with significant reduction in cell viability and increased cell apoptosis and inflammatory cytokines levels, whereas opposite results were observed in NFIB inhibition treatment group. Moreover, RIP2 was up-regulated by NFIB overexpression, and RIP2 silence mitigated the effect of NFIB overexpression in cell apoptosis, inflammation, and activation of NFκB signaling pathways. This study demonstrated that NFIB overexpression accelerated APEC-induced apoptosis and inflammation via up-regulation of RIP2 mediated downstream pathways in chicken HD11 cells.
Keywords: APEC; NFIB; RIP2; apoptosis; gene expression; immune response.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





Similar articles
-
Gene expression profiling of RIP2-knockdown in HD11 macrophages - elucidation of potential pathways (gene network) when challenged with avian pathogenic E.coli (APEC).BMC Genomics. 2022 May 2;23(1):341. doi: 10.1186/s12864-022-08595-5. BMC Genomics. 2022. PMID: 35501708 Free PMC article.
-
Analysis of miRNA Expression Profiling of RIP2 Knockdown in Chicken HD11 Cells When Infected with Avian Pathogenic E. coli (APEC).Int J Mol Sci. 2022 Jun 30;23(13):7319. doi: 10.3390/ijms23137319. Int J Mol Sci. 2022. PMID: 35806321 Free PMC article.
-
Long noncoding RNAs expression profile of RIP2 knockdown in chicken HD11 macrophages associated with avian pathogenic E. coli (APEC) infection.Dev Comp Immunol. 2023 May;142:104650. doi: 10.1016/j.dci.2023.104650. Epub 2023 Feb 2. Dev Comp Immunol. 2023. PMID: 36736641
-
Outer membrane vesicles of avian pathogenic Escherichia coli induce necroptosis and NF-κB activation in chicken macrophages via RIPK1 mediation.Res Vet Sci. 2024 Apr;170:105185. doi: 10.1016/j.rvsc.2024.105185. Epub 2024 Feb 13. Res Vet Sci. 2024. PMID: 38422838
-
Nuclear factor I/B: Duality in action in cancer pathophysiology.Cancer Lett. 2025 Jan 28;609:217349. doi: 10.1016/j.canlet.2024.217349. Epub 2024 Nov 22. Cancer Lett. 2025. PMID: 39581218 Review.
Cited by
-
Gene expression profiling of RIP2-knockdown in HD11 macrophages - elucidation of potential pathways (gene network) when challenged with avian pathogenic E.coli (APEC).BMC Genomics. 2022 May 2;23(1):341. doi: 10.1186/s12864-022-08595-5. BMC Genomics. 2022. PMID: 35501708 Free PMC article.
-
Transcriptional regulation of NDUFA4L2 by NFIB induces sorafenib resistance by decreasing reactive oxygen species in hepatocellular carcinoma.Cancer Sci. 2023 Mar;114(3):793-805. doi: 10.1111/cas.15648. Epub 2022 Nov 29. Cancer Sci. 2023. PMID: 36369883 Free PMC article.
-
Genome-wide transcriptional profiling and functional analysis of long noncoding RNAs and mRNAs in chicken macrophages associated with the infection of avian pathogenic E. coli.BMC Vet Res. 2024 Feb 7;20(1):49. doi: 10.1186/s12917-024-03890-7. BMC Vet Res. 2024. PMID: 38326918 Free PMC article.
-
Identification and Functional Analysis of Novel Long Intergenic RNA in Chicken Macrophages Infected with Avian Pathogenic Escherichia coli.Microorganisms. 2024 Aug 6;12(8):1594. doi: 10.3390/microorganisms12081594. Microorganisms. 2024. PMID: 39203441 Free PMC article.
-
Editorial of Special Issue "Roles of Inflammasomes and Methyltransferases in Inflammation".Int J Mol Sci. 2022 Sep 7;23(18):10283. doi: 10.3390/ijms231810283. Int J Mol Sci. 2022. PMID: 36142195 Free PMC article.
References
-
- Nolan L.K., John B.H., Vaillancourt J.P., Abdul-Aziz T., Logue C.M. Colibacillosis. In: Swayne D.E., editor. Diseases of Poultry. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2017. pp. 751–805.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

