Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability
- PMID: 35409192
- PMCID: PMC8998954
- DOI: 10.3390/ijms23073832
Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability
Abstract
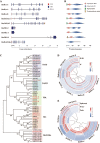
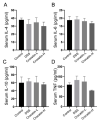
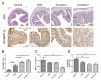
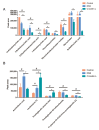
Crocetin is one of the major active constituents of saffron (Crocus sativus L.) which has a reputation for facilitating blood circulation and dispersing blood stasis in traditional Chinese medicine. However, there is little evidence showing the relationship between crocetin intake and the risk of gastrointestinal diseases such as colitis. In order to investigate the effect of crocetin on the regulation of intestinal barrier function and intestinal microbiota composition, mice were treated with crocetin after 3% dextran sulfate sodium (DSS) administration for one week. We found that crocetin intake at 10 mg/kg aggravated colitis in mice, showing increased weight loss and more serious histological abnormalities compared with the DSS group. The 16s rDNA sequencing analysis of the feces samples showed that mice treated with 10 mg/kg crocetin had lower species diversity and richness than those treated with DSS. At the genus level, a higher abundance of Akkermansia and Mediterraneibacter, and a lower abundance of Muribaculaceae, Dubosiella, Paramuribaculum, Parasutterella, Allobaculum, Duncaniella, Candidatus Stoquefichus, and Coriobacteriaceae UCG-002 were observed in the crocetin group. Untargeted metabolomic analyses revealed that crocetin reduced the levels of primary and secondary bile acids such as 12-ketodeoxycholic acid, 7-ketodeoxycholic acid, 3-sulfodeoxycholic acid, 6-ethylchenodeoxycholic acid, chenodeoxycholate, glycochenodeoxycholate-7-sulfate, glycocholate, and sulfolithocholic acid in the colon. In conclusion, crocetin intake disturbed intestinal homeostasis and prolonged recovery of colitis by promoting inflammation and altering gut microbiota composition and its metabolic products in mice. Our findings suggest that patients with gastrointestinal diseases such as inflammatory bowel disease should use crocetin with caution.
Keywords: crocetin; gut microbiota; inflammatory bowel disease; intestinal metabolites; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J., Chan F., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

